Xanthine dehydrogenase
Xanthine dehydrogenase, also known as XDH, is a protein that, in humans, is encoded by the XDH gene.[5][6]
| xanthine dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.17.1.4 | ||||||||
| CAS number | 9054-84-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Function
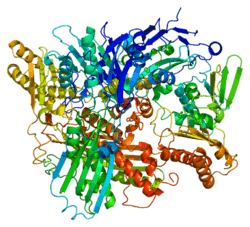
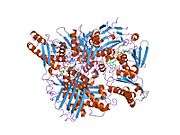
Xanthine dehydrogenase belongs to the group of molybdenum-containing hydroxylases involved in the oxidative metabolism of purines. The enzyme is a homodimer. Xanthine dehydrogenase can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification.[5]
Xanthine dehydrogenase catalyzes the following chemical reaction:
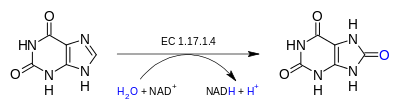
The three substrates of this enzyme are xanthine, NAD+, and H2O, whereas its three products are urate, NADH, and H+.
This enzyme participates in purine metabolism.
Nomenclature
This enzyme belongs to the family of oxidoreductases, to be specific, those acting on CH or CH2 groups with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is xanthine:NAD+ oxidoreductase. Other names in common use include NAD+-xanthine dehydrogenase, xanthine-NAD+ oxidoreductase, xanthine/NAD+ oxidoreductase, and xanthine oxidoreductase.
Clinical significance
Defects in xanthine dehydrogenase cause xanthinuria, may contribute to adult respiratory stress syndrome, and may potentiate influenza infection through an oxygen metabolite-dependent mechanism.[5] It has been shown that patients with lung adenocarcinoma tumors which have high levels of XDH gene expression, have lower survivals.[7][8] Addiction to XDH protein has been used to target NSCLC tumors and cell lines in a precision oncology manner.[8]
References
- GRCh38: Ensembl release 89: ENSG00000158125 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024066 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: XDH xanthine dehydrogenase". Retrieved October 25, 2012.
- Ichida K, Amaya Y, Noda K, Minoshima S, Hosoya T, Sakai O, Shimizu N, Nishino T (November 1993). "Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene". Gene. 133 (2): 279–84. doi:10.1016/0378-1119(93)90652-J. PMID 8224915.
- Konno H, Minamiya Y, Saito H, Imai K, Kawaharada Y, Motoyama S, Ogawa J (October 2012). "Acquired xanthine dehydrogenase expression shortens survival in patients with resected adenocarcinoma of lung". Tumour Biology. 33 (5): 1727–32. doi:10.1007/s13277-012-0431-2. PMID 22678977.
- Tavassoly I, Hu Y, Zhao S, Mariottini C, Boran A, Chen Y, Li L, Tolentino RE, Jayaraman G, Goldfarb J, Gallo J, Iyengar R (May 2019). "Genomic signatures defining responsiveness to allopurinol and combination therapy for lung cancer identified by systems therapeutics analyses". Molecular Oncology. 13 (8): 1725–1743. doi:10.1002/1878-0261.12521. PMC 6670022. PMID 31116490.
Further reading
- Battelli MG, Lorenzoni E (October 1982). "Purification and properties of a new glutathione-dependent thiol:disulphide oxidoreductase from rat liver". The Biochemical Journal. 207 (1): 133–8. doi:10.1042/bj2070133. PMC 1153833. PMID 6960894.
- Corte ED, Stirpe F (February 1972). "The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme". The Biochemical Journal. 126 (3): 739–45. doi:10.1042/bj1260739. PMC 1178433. PMID 4342395.
- Parzen SD, Fox AS (December 1964). "Purification of xanthine dehydrogenase from Drosophila melanogaster". Biochimica et Biophysica Acta (BBA) - Specialized Section on Enzymological Subjects. 92 (3): 465–71. doi:10.1016/0926-6569(64)90006-9. PMID 14264879.
- Rajagopalan KV, Handler P (September 1967). "Purification and properties of chicken liver xanthine dehydrogenase". The Journal of Biological Chemistry. 242 (18): 4097–107. PMID 4294045.
- Smith ST, Rajagopalan KV, Handler P (September 1967). "Purification and properties of xanthine dehydroganase from Micrococcus lactilyticus". The Journal of Biological Chemistry. 242 (18): 4108–17. PMID 6061702.
- Parschat K, Canne C, Hüttermann J, Kappl R, Fetzner S (January 2001). "Xanthine dehydrogenase from Pseudomonas putida 86: specificity, oxidation-reduction potentials of its redox-active centers, and first EPR characterization". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1544 (1–2): 151–65. doi:10.1016/S0167-4838(00)00214-4. PMID 11341925.
- Ichida K, Amaya Y, Noda K, Minoshima S, Hosoya T, Sakai O, Shimizu N, Nishino T (November 1993). "Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene". Gene. 133 (2): 279–84. doi:10.1016/0378-1119(93)90652-J. PMID 8224915.
- Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF (September 2000). "Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion". Proceedings of the National Academy of Sciences of the United States of America. 97 (20): 10723–8. Bibcode:2000PNAS...9710723E. doi:10.1073/pnas.97.20.10723. PMC 27090. PMID 11005854.
- Truglio JJ, Theis K, Leimkühler S, Rappa R, Rajagopalan KV, Kisker C (January 2002). "Crystal structures of the active and alloxanthine-inhibited forms of xanthine dehydrogenase from Rhodobacter capsulatus". Structure. 10 (1): 115–25. doi:10.1016/S0969-2126(01)00697-9. PMID 11796116.
- Hille R (November 1996). "The Mononuclear Molybdenum Enzymes". Chemical Reviews. 96 (7): 2757–2816. doi:10.1021/cr950061t. PMID 11848841.
- Meneshian A, Bulkley GB (July 2002). "The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction". Microcirculation. 9 (3): 161–75. doi:10.1038/sj.mn.7800136. PMID 12080414.
- Xu P, Huecksteadt TP, Harrison R, Hoidal JR (October 1995). "Molecular cloning, tissue expression of human xanthine dehydrogenase". Biochemical and Biophysical Research Communications. 215 (1): 429. doi:10.1006/bbrc.1995.2482. PMID 7575623.
- Xu P, Zhu XL, Huecksteadt TP, Brothman AR, Hoidal JR (September 1994). "Assignment of human xanthine dehydrogenase gene to chromosome 2p22". Genomics. 23 (1): 289–91. doi:10.1006/geno.1994.1498. PMID 7829092.
- Minoshima S, Wang Y, Ichida K, Nishino T, Shimizu N (1994). "Mapping of the gene for human xanthine dehydrogenase (oxidase) (XDH) to band p23 of chromosome 2". Cytogenetics and Cell Genetics. 68 (1–2): 52–3. doi:10.1159/000133887. PMID 7956358.
- Rytkönen EM, Halila R, Laan M, Saksela M, Kallioniemi OP, Palotie A, Raivio KO (1994). "The human gene for xanthine dehydrogenase (XDH) is localized on chromosome band 2q22". Cytogenetics and Cell Genetics. 68 (1–2): 61–3. doi:10.1159/000133890. PMID 7956361.
- Xu P, Huecksteadt TP, Harrison R, Hoidal JR (March 1994). "Molecular cloning, tissue expression of human xanthine dehydrogenase". Biochemical and Biophysical Research Communications. 199 (2): 998–1004. doi:10.1006/bbrc.1994.1328. PMID 8135849.
- Ichida K, Amaya Y, Noda K, Minoshima S, Hosoya T, Sakai O, Shimizu N, Nishino T (November 1993). "Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene". Gene. 133 (2): 279–84. doi:10.1016/0378-1119(93)90652-J. PMID 8224915.
- Satoh A, Sasago S, Takahashi S, Kato N (September 1993). "Regulation of xanthine dehydrogenase in rat liver in response to peroxisome proliferators". Biochemical and Biophysical Research Communications. 195 (2): 751–7. doi:10.1006/bbrc.1993.2109. PMID 8373410.
- Xu P, Huecksteadt TP, Hoidal JR (June 1996). "Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH)". Genomics. 34 (2): 173–80. doi:10.1006/geno.1996.0262. PMID 8661045.
- Saksela M, Raivio KO (April 1996). "Cloning and expression in vitro of human xanthine dehydrogenase/oxidase". The Biochemical Journal. 315 (Pt 1): 235–9. doi:10.1042/bj3150235. PMC 1217176. PMID 8670112.
- Many A, Westerhausen-Larson A, Kanbour-Shakir A, Roberts JM (1996). "Xanthine oxidase/dehydrogenase is present in human placenta". Placenta. 17 (5–6): 361–5. doi:10.1016/S0143-4004(96)90061-2. PMID 8829220.
- Hellsten Y, Frandsen U, Orthenblad N, Sjødin B, Richter EA (January 1997). "Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation". The Journal of Physiology. 498 (Pt 1): 239–48. doi:10.1113/jphysiol.1997.sp021855. PMC 1159248. PMID 9023782.
- Ichida K, Amaya Y, Kamatani N, Nishino T, Hosoya T, Sakai O (May 1997). "Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria". The Journal of Clinical Investigation. 99 (10): 2391–7. doi:10.1172/JCI119421. PMC 508078. PMID 9153281.
- Rouquette M, Stevens C, Blake DR, Harrison R, Whish J, Whish WD (August 1997). "Expression of xanthine oxidase activity in human endothelial cells as a function of cell density". Biochemical Society Transactions. 25 (3): 532S. doi:10.1042/bst025532s. PMID 9388748.
- Newaz MA, Adeeb NN (March 1998). "Detection of xanthine oxidase in human plasma". The Medical Journal of Malaysia. 53 (1): 70–5. PMID 10968141.
- Martelin E, Palvimo JJ, Lapatto R, Raivio KO (September 2000). "Nuclear factor Y activates the human xanthine oxidoreductase gene promoter". FEBS Letters. 480 (2–3): 84–8. doi:10.1016/S0014-5793(00)01909-8. PMID 11034305.
- Stiborová M, Frei E, Sopko B, Wiessler M, Schmeiser HH (April 2002). "Carcinogenic aristolochic acids upon activation by DT-diaphorase form adducts found in DNA of patients with Chinese herbs nephropathy". Carcinogenesis. 23 (4): 617–25. doi:10.1093/carcin/23.4.617. PMID 11960915.
- Frederiks WM, Vreeling-Sindelárová H (2002). "Ultrastructural localization of xanthine oxidoreductase activity in isolated rat liver cells". Acta Histochemica. 104 (1): 29–37. doi:10.1078/0065-1281-00629. PMID 11993848.
- Cejková J, Ardan T, Filipec M, Midelfart A (2003). "Xanthine oxidoreductase and xanthine oxidase in human cornea". Histology and Histopathology. 17 (3): 755–60. doi:10.14670/HH-17.755. PMID 12168784.