Water activity
Water activity (aw) is the partial vapor pressure of water in a substance divided by the standard state partial vapor pressure of water. In the field of food science, the standard state is most often defined as the partial vapor pressure of pure water at the same temperature. Using this particular definition, pure distilled water has a water activity of exactly one. As temperature increases, aw typically increases, except in some products with crystalline salt or sugar.
| Food safety |
|---|
 |
| Terms |
| Critical factors |
| Bacterial pathogens |
| Viral pathogens |
| Parasitic pathogens |
Higher aw substances tend to support more microorganisms.
Water migrates from areas of high aw to areas of low aw. For example, if honey (aw ≈ 0.6) is exposed to humid air (aw ≈ 0.7), the honey absorbs water from the air. If salami (aw ≈ 0.87) is exposed to dry air (aw ≈ 0.5), the salami dries out, which could preserve it or spoil it.
Formula
Definition of aw:
where p is the partial vapor pressure of water in the solution, and p* is the partial vapor pressure of pure water at the same temperature.
Alternate definition:
where lw is the activity coefficient of water and xw is the mole fraction of water in the aqueous fraction.
Relationship to relative humidity: The relative humidity of air in equilibrium with a sample is called the Equilibrium Relative Humidity (ERH).[1]
Estimated mold-free shelf life in days at 21° C:
Uses
Water activity is an important consideration for food product design and food safety.
Food product design
Food designers use water activity to formulate shelf-stable food. If a product is kept below a certain water activity, then mold growth is inhibited. This results in a longer shelf life.
Water activity values can also help limit moisture migration within a food product made with different ingredients. If raisins of a higher water activity are packaged with bran flakes of a lower water activity, the water from the raisins migrates to the bran flakes over time, making the raisins hard and the bran flakes soggy. Food formulators use water activity to predict how much moisture migration affects their product.
Food safety
Water activity is used in many cases as a critical control point for Hazard Analysis and Critical Control Points (HACCP) programs. Samples of the food product are periodically taken from the production area and tested to ensure water activity values are within a specified range for food quality and safety. Measurements can be made in as little as five minutes, and are made regularly in most major food production facilities.
For many years, researchers tried to equate bacterial growth potential with water content. They found that the values were not universal, but specific to each food product. W. J. Scott first established that bacterial growth correlated with water activity, not water content, in 1953. It is firmly established that growth of bacteria is inhibited at specific water activity values. U.S. Food and Drug Administration (FDA) regulations for intermediate moisture foods are based on these values.
Lowering the water activity of a food product should not be seen as a kill step. Studies in powdered milk show that viable cells can exist at much lower water activity values, but that they never grow. Over time, bacterial levels decline.
Measurement
Water activity values are obtained by either a resistive electrolytic, a capacitance or a dew point hygrometer.
Resistive electrolytic hygrometers
Resistive electrolytic hygrometers use a sensing element in the form of a liquid electrolyte held in between of two small glass rods by capillary force. The electrolyte changes resistance if it absorbs or loses water vapor. The resistance is directly proportional to relative air humidity, and also to water activity of the sample (once vapor–liquid equilibrium is established). This relation can be checked by either a verification or calibration using salt-water mixtures, which provide a well-defined and reproducible air humidity in the measurement chamber.
The sensor does not have any physically given hysteresis as it is known from capacitance hygrometers and sensors, and does not require regular cleaning as its surface is not the effectively sensing element. Volatiles, in principle, influence the measurement performance—especially those that dissociate in the electrolyte and thereby change its resistance. Such influences can easily be avoided by using chemical protection filters that absorb the volatile compound before arriving at the sensor.
Capacitance hygrometers
Capacitance hygrometers consist of two charged plates separated by a polymer membrane dielectric. As the membrane adsorbs water, its ability to hold a charge increases and the capacitance is measured. This value is roughly proportional to the water activity as determined by a sensor-specific calibration.
Capacitance hygrometers are not affected by most volatile chemicals and can be much smaller than other alternative sensors. They do not require cleaning, but are less accurate than dew point hygrometers (+/- 0.015 aw). They should have regular calibration checks and can be affected by residual water in the polymer membrane (hysteresis).
Dew point hygrometers
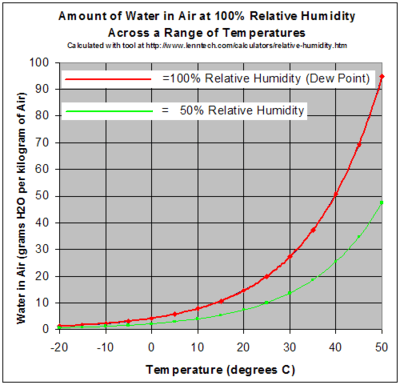
The temperature at which dew forms on a clean surface is directly related to the vapor pressure of the air. Dew point hygrometers work by placing a mirror over a closed sample chamber. The mirror is cooled until the dew point temperature is measured by means of an optical sensor. This temperature is then used to find the relative humidity of the chamber using psychrometrics charts.
This method is theoretically the most accurate (+/- 0.003 aw) and often the fastest. The sensor requires cleaning if debris accumulates on the mirror.
Equilibration
With either method, vapor–liquid equilibrium must occur in the sample chamber. This takes place over time or can be aided by the addition of a fan in the chamber. Thermal equilibrium must also take place unless the sample temperature is measured.
Moisture content
Water activity is related to water content in a non-linear relationship known as a moisture sorption isotherm curve. These isotherms are substance- and temperature-specific. Isotherms can be used to help predict product stability over time in different storage conditions.
Use in humidity control
There is net evaporation from a solution with a water activity greater than the relative humidity of its surroundings. There is net absorption of water by a solution with a water activity less than the relative humidity of its surroundings. Therefore, in an enclosed space, a solution can be used to regulate humidity.[3]
Selected aw values
| Substance | aw | Source |
|---|---|---|
| Distilled Water | 1.00 | [4] |
| Tap water | 0.99 | |
| Raw meats | 0.99 | [4] |
| Milk | 0.97 | |
| Juice | 0.97 | |
| Salami | 0.87 | [4] |
| Shelf-stable cooked bacon | < 0.85 | [5] |
| Saturated NaCl solution | 0.75 | |
| Point at which cereal loses crunch | 0.65 | |
| Dried fruit | 0.60 | [4] |
| Typical indoor air | 0.5 - 0.7 | |
| Honey | 0.5 - 0.7 | |
| Peanut Butter | ≤ 0.35 | [6] |
| Microorganism Inhibited | aw | Source |
|---|---|---|
| Clostridium botulinum E | 0.97 | [7] |
| Pseudomonas fluorescens | 0.97 | [7] |
| Clostridium perfringens | 0.95 | [7] |
| Escherichia coli | 0.95 | [7] |
| Clostridium botulinum A, B | 0.94 | [7] |
| Salmonella | 0.93 | [8] |
| Vibrio cholerae | 0.95 | [7] |
| Bacillus cereus | 0.93 | [7] |
| Listeria monocytogenes | 0.92, (0.90 in 30% glycerol) | [9] |
| Bacillus subtilis | 0.91 | [7] |
| Staphylococcus aureus | 0.86 | [10] |
| Most molds | 0.80 | [10] |
| No microbial proliferation | <0.60 | [7] |
References
- Young, Linda; Cauvain, Stanley P. (2000). Bakery food manufacture and quality: water control and effects. Oxford: Blackwell Science. ISBN 978-0-632-05327-8.
- Man, C.M.D.; Jones, Adrian A. (2000). Shelf Life Evaluation of Foods. Springer. ISBN 978-0-834-21782-9.
- Demchick, P. H. (1984). "Taking control of chamber humidity". The Science Teacher. 51 (7): 29‑31.
- Marianski, Stanley; Marianski, Adam (2008). The Art of Making Fermented Sausages. Denver, Colorado: Outskirts Press. ISBN 978-1-4327-3257-8.
- "Bacon and Food Safety". United States Department of Agriculture Food Safety and Inspection Service. 2013-10-29. Retrieved 2017-06-18.
- He, Y.; Li, Y.; Salazar, J. K.; Yang, J.; Tortorello, M. L.; Zhang, W. (2013). "Increased Water Activity Reduces the Thermal Resistance of Salmonella enterica in Peanut Butter". Applied and Environmental Microbiology. 79 (15): 4763–4767. doi:10.1128/AEM.01028-13. PMC 3719514. PMID 23728806.
- Barbosa-Canovas, G.; Fontana, A.; Schmidt, S.; Labuza, T.P. (2007). "Appendix D: Minimum Water Activity Limits for Growth of Microorganisms". Water Activity in Foods: Fundamentals and Applications. FT Blackwell Press. pp. Appendix D. doi:10.1002/9780470376454.app4. ISBN 9780470376454.
- Shaw, Angela (2013). Salmonella: Create the most undesirable environment. Ames, IA: Iowa State University.
- Ryser, Elliot T.; Elmer, Marth H. (2007). Listeria, Listeriosis and Food Safety (3rd ed.). CRC Press. pp. 173–174.
- Marianski, 7
- Reineccius, Gary (1998). Sourcebook of Flavors. Berlin: Springer. ISBN 978-0-8342-1307-4.
- Fennema, O.R., ed. (1985). Food Chemistry (2nd ed.). New York: Marcell Dekker, Inc. pp. 46–50.
- Bell, L.N.; Labuza, T.P. (2000). Practical Aspects of Moisture Sorption Isotherm Measurement and Use (2nd ed.). Egan, MN: AACC Egan Press.
External links
- Isotopic effect
- Measurement
- Why measure water activity?, Syntilab
- How to measure water activity?, Syntilab