Tioconazole
Tioconazole is an antifungal medication of the imidazole class used to treat infections caused by a fungus or yeast. It is marketed under the brand names Trosyd and Gyno-Trosyd (Pfizer, now Johnson & Johnson). Tioconazole ointments serve to treat women's vaginal yeast infections.[1] They are available in one day doses, as opposed to the 7-day treatments more common in use in the past.
 | |
| Clinical data | |
|---|---|
| Trade names | Vagistat-1 |
| Other names | Thioconazole |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.059.958 |
| Chemical and physical data | |
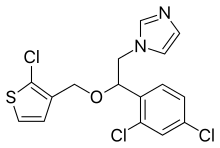
| Formula | C16H13Cl3N2OS |
| Molar mass | 387.70 g·mol−1 |
| Chirality | Racemic mixture |
| | |
Tioconazole topical (skin) preparations are also available for ringworm, jock itch, athlete's foot, and tinea versicolor or "sun fungus".
It was patented in 1975 and approved for medical use in 1982.[2]
Side effects
Side effects (for the women's formulas) may include temporary burning/irritation of the vaginal area, moderate drowsiness, headache similar to a sinus headache, hives, and upper respiratory infection. These side effects may be only temporary, and do not normally interfere with the patient's comfort enough to outweigh the end result.
Synthesis
Antimycotic imidazole derivative.

A displacement reaction between 1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethanol and 2-chloro-3-(chloromethyl)thiophene is performed.
References
- Tioconazole, Mayo Clinic
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 503. ISBN 9783527607495.
- G. E. Gymer, BE 841309; idem, U.S. Patent 4,062,966 (1976, 1977 both to Pfizer).
| Wikimedia Commons has media related to Tioconazole. |