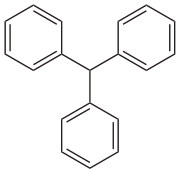
Triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical).
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1′,1′′-Methanetriyltribenzene | |
| Other names
Triphenylmethane 1,1′,1′′-Methylidynetrisbenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.524 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H16 | |
| Molar mass | 244.337 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.014 g/cm3 |
| Melting point | 92 to 94 °C (198 to 201 °F; 365 to 367 K) |
| Boiling point | 359 °C (678 °F; 632 K) |
| insoluble | |
| Solubility | soluble in dioxane[1] and hexane |
| Acidity (pKa) | 33.3 |
| -165.6·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | External MSDS |
| R-phrases (outdated) | R36 R37 R38 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Triphenylmethane was first synthesized in 1872 by the German chemist August Kekulé and his Belgian student Antoine Paul Nicolas Franchimont (1844–1919) by heating diphenylmercury (Hg(C6H5)2, Quecksilberdiphenyl) with benzal chloride (C6H5CHCl2, Benzylenchlorid).[2]
Triphenylmethane can be synthesized by Friedel-Crafts reaction from benzene and chloroform with aluminium chloride catalyst:
- 3 C6H6 + CHCl3 → Ph3CH + 3 HCl
Alternatively, benzene may react with carbon tetrachloride using the same catalyst to obtain the trityl chloride-aluminium chloride adduct, which is hydrolyzed with dilute acid:[3]
- 3 C6H6 + CCl4 + AlCl3 → Ph3CCl·AlCl3
- Ph3CCl·AlCl3 + HCl → Ph3CH
Synthesis from benzylidene chloride, prepared from benzaldehyde and phosphorus pentachloride, is used as well.
Acidity
The pKa is 33.3.[4] Triphenylmethane is significantly more acidic than most other hydrocarbons because the charge is delocalized over three phenyl rings. Steric effects however prevent all three phenyl rings from achieving coplanarity simultaneously. Consequently diphenylmethane is even more acidic, because in its anion the charge is spread over two phenyl rings at the same time. The trityl anion is red. This colour can be used as an indicator in acid-base titrations.
The sodium salt can be prepared also from the chloride:[5]
- (C6H5)3CCl + 2 Na → (C6H5)3CNa + NaCl
The use of tritylsodium as a strong, non-nucleophilic base has been eclipsed by the popularization of butyllithium and related strong bases.
Triarylmethane dyes
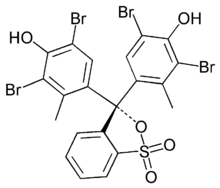
Examples of triarylmethane dyes are bromocresol green:
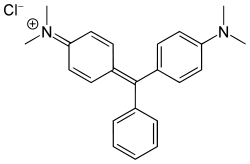
And the nitrogen-bearing malachite green:
See also
References
- "Triphenylmethane | 519-73-3".
- Aug. Kekulé and A. Franchimont (1872) "Ueber das Triphenylmethan" (On triphenylmethane), Berichte der deutschen chemischen Gesellschaft, 5 : 906–908.
- J. F. Norris (1925). "Triphenylmethane". 4: 81. doi:10.15227/orgsyn.004.0081. Cite journal requires
|journal=(help) - Ronald Breslow and William Chu (1969). "Electrochemical determinations of pKa's. Triphenylmethanes and cycloheptatriene". Journal of the American Chemical Society. 92 (7): 2165. doi:10.1021/ja00710a077.
- W. B. Renfrow Jr and C. R. Hauser (1943). "Triphenylmethylsodium". Organic Syntheses.; Collective Volume, 2, p. 607