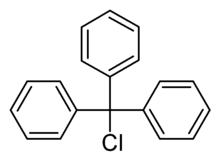
Triphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1′,1″-(Chloromethanetriyl)tribenzene | |||
| Other names
(Chloromethanetriyl)tribenzene [Chloro(diphenyl)methyl]benzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.898 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C19H15Cl | |||
| Molar mass | 278.7754 g/mol | ||
| Appearance | white to yellow solid | ||
| Density | 1.141 g/cm3 | ||
| Melting point | 109 to 112 °C (228 to 234 °F; 382 to 385 K) | ||
| Boiling point | 230 °C (446 °F; 503 K) (at 20 mmHg) and 374.3 °C (at 760 mmHg) | ||
| Solubility | soluble in chloroform, benzene, acetone,[1] ether, THF, hexane[2] | ||
| Hazards | |||
| Safety data sheet | Corvine Chemicals MSDS | ||
| Flash point | 177.9 °C (352.2 °F; 451.0 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Triphenylmethyl chloride is commercially available. It may be prepared by the reaction of triphenylmethanol with acetyl chloride, or by the Friedel-Crafts alkylation of benzene with carbon tetrachloride to give the trityl chloride-aluminium chloride adduct, which is then hydrolyzed.[3]
Reactions
Triphenylmethylsodium can be prepared from trityl chloride dissolved in an aprotic solvent and sodium:[4]
- (C6H5)3CCl + 2 Na → (C6H5)3CNa + NaCl
Reaction with silver hexafluorophosphate gives triphenylmethyl hexafluorophosphate.
gollark: Why not?
gollark: It's not like I can't just pass it off to GTech™ bee neuron intelligences.
gollark: Maybe *I* am to learn Toki Pona.
gollark: This would be even more ethical.
gollark: Wait, what if you just raise them on provably sound and formally verified languages?
See also
References
- http://www.sciencelab.com/msds.php?msdsId=9925340
- https://www.scbt.com/scbt/product/trityl-chloride-76-83-5
- W. E. Bachmann; C. R. Hauser; Boyd E. Hudson, Jr. (1955). "Triphenylchloromethane". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 841
- W. B. Renfrow Jr and C. R. Hauser (1943). "Triphenylmethylsodium". Organic Syntheses.; Collective Volume, 2, p. 607
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.