Benzal chloride
Benzal chloride is an organic compound with the formula C6H5CHCl2.[1] This colourless liquid is a lachrymator and is used as a building block in organic synthesis.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Dichloromethyl)benzene | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1099407 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.463 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Compounds Benzylidene Compounds | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1886 2810 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6Cl2 | |||
| Molar mass | 161.03 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.254 g/cm3, liquid | ||
| Melting point | −17 to −15 °C (1 to 5 °F; 256 to 258 K) | ||
| Boiling point | 205 °C (401 °F; 478 K) (82 °C at 10 mmHg) | ||
| 0.25 g/L at 39 °C | |||
| Vapor pressure | 0.6 kPa (45 °C) | ||
| Hazards | |||
| GHS pictograms | 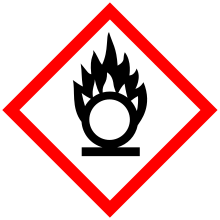    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H302, H315, H318, H331, H335, H351 | ||
| P201, P202, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P304+340, P305+351+338, P308+313, P310, P311, P312, P321, P330, P332+313, P362, P403+233, P405, P501 | |||
| Flash point | 93 °C (199 °F; 366 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and usage
Benzal chloride is produced by the free radical chlorination of toluene, being preceded in the process by benzyl chloride (C6H5CH2Cl) and followed by benzotrichloride (C6H5CCl3):
- C6H5CH3 + Cl2 → C6H5CH2Cl + HCl
- C6H5CH2Cl + Cl2 → C6H5CHCl2 + HCl
- C6H5CHCl2 + Cl2 → C6H5CCl3 + HCl
Benzylic halides are typically strong alkylating agents, and for this reason benzal chloride is treated as a hazardous compound.
Treatment of benzal chloride with sodium gives stilbene.
Most benzal chloride main industrial use is as a precursor to benzaldehyde. This conversion involves hydrolysis in the presence of base:[2]
- C6H5CHCl2 + H2O → C6H5CHO + 2 HCl
gollark: Aha, I knew it! `dmesg` shows a bunch of errors roughly coinciding with the freezing/crashing.
gollark: It's not a proxy.
gollark: Also, it might be something other than nvidia's fault, dare I say.
gollark: Unfortunately, AMD's price/performance is a bit awful currently.
gollark: Stupid mysterious freezing and crashing. I blame nvidia.
References
- "BENZAL CHLORIDE". International Programme on Chemical Safety. Retrieved 2007-10-30.
- Lipper, Karl-August; Löser, Eckhard. "Benzyl Chloride and Other Side-Chain Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o04_o01.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.