Translocon
The translocon (commonly known as a translocator or translocation channel) is a complex of proteins associated with the translocation of polypeptides across membranes.[1] In eukaryotes the term translocon most commonly refers to the complex that transports nascent polypeptides with a targeting signal sequence into the interior (cisternal or lumenal) space of the endoplasmic reticulum (ER) from the cytosol. This translocation process requires the protein to cross a hydrophobic lipid bilayer. The same complex is also used to integrate nascent proteins into the membrane itself (membrane proteins). In prokaryotes, a similar protein complex transports polypeptides across the (inner) plasma membrane or integrates membrane proteins.[2] Bacterial pathogens can also assemble other translocons in their host membranes, allowing them to export virulence factors into their target cells.[3]
In either case, the protein complex are formed from Sec proteins (Sec: secretory), with the hetrotrimeric Sec61 being the channel.[4] In prokaryotes, the homologous channel complex is known as SecYEG.[5]
The central channel
The translocation channel is a hetero-trimeric protein complex called SecYEG in prokaryotes and Sec61 in eukaryotes. It consists of the subunits SecY, SecE, and SecG. The structure of this channel, in its idle state, has been solved by X-ray crystallography in archaea.[5] SecY is the large pore subunit. In a side view, the channel has an hourglass shape, with a funnel on each side. The extracellular funnel has a little "plug" formed out of an alpha-helix. In the middle of the membrane is a construction, formed from a pore ring of six hydrophobic amino acids that project their side chains inwards. During protein translocation, the plug is moved out of the way, and a polypeptide chain is moved from the cytoplasmic funnel, through the pore ring, the extracellular funnel, into the extracellular space. Hydrophobic segments of membrane proteins exit sideways through the lateral gate into the lipid phase and become membrane-spanning segments.[5]
In E. coli, SecYEG complexes dimerize on the membrane.[6] In eukaryotes, multiple copies of Sec61 come together and form a larger complex along with further components such as the oligosaccharyl transferase complex, the TRAP complex, and the membrane protein TRAM (possible chaperone). For further components, such as signal peptidase complex and the SRP receptor it is not clear to what extent they only associate transiently to the translocon complex.[7]
Translocation
The channel allows peptides to move in either direction, so additional systems in the translocon are required to move the peptide in a specific direction. There are three types of translocation: cotranslational translocation that happens as translation happens, and two types of post-translational translocation that happens after translation, each seen in eukaryotes and bacteria. While eukaryotes unfold the protein with BiP and use other complexes to transport the peptide, bacteria use the SecA ATPase.[8]
Co-translational
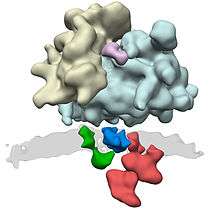
In co-translational translocation, the translocon associates with the ribosome so that a growing nascent polypeptide chain is moved from the ribosome tunnel into the SecY channel. The translocon (translocator) acts as a channel through the hydrophobic membrane of the endoplasmic reticulum (after the SRP has dissociated and translation is continued). The emerging polypeptide is threaded through the channel as an unfolded string of amino acids, potentially driven by a Brownian Ratchet. Once translation is finished, a signal peptidase cleaves off the short signal peptide from the nascent protein, leaving the polypeptide free in the interior of the endoplasmic reticulum.[9][10]
In eukaryotes, proteins due to be translocated to the endoplasmic reticulum are recognized by the signal-recognition particle (SRP), which halts translation of the polypeptide by the ribosome while it attaches the ribosome to the SRP receptor on the endoplasmic reticulum. This recognition event is based upon a specific N-terminal signal sequence that is in the first few codons of the polypeptide to be synthesised.[8] Bacteria also use an SRP, together with a chaperone YidC that is similar to the eukaryote TRAM.[11][8]
The translocon can also translocate and integrate membrane proteins in the correct orientation into the membrane of the endoplasmic reticulum. The mechanism of this process is not fully understood, but involves the recognition and processing by the translocon of hydrophobic stretches in the amino acid sequence which are destined to become transmembrane helices. Closed by stop-transfer sequences and opened by embedded signal sequences, the plug alters between its open and closed states to place helices in different orientations.[8]
Post-translational
In eukaryotes, post-translational translocation depends on BiP and other complexes, including the SEC62/SEC63 integral membrane protein complex. In this mode of translocation, Sec63 helps BiP hydrolyze ATP, which then binds to the peptide and "pulls" it out. This process is repeated for other BiP molecules until the entire peptide has been pulled through.[8]
In bacteria, the same process is done by a "pushing" ATPase known as SecA, sometimes assisted by the SecDF complex on the other side responsible for pulling.[12] The SecA ATPase uses a "push-and-slide" mechanism to move a polypeptide through the channel. In the ATP-bound state, SecA interacts through a two-helix finger with a subset of amino acids in a substrate, pushing them (with ATP hydrolysis) into the channel. The interaction is then weakened as SecA enters the ADP-bound state, allowing the polypeptide chain to slide passively in either direction. SecA then grabs a further section of the peptide to repeat the process.[8]
The ER-retrotranslocon
Translocators can also move polypeptides (such as damaged proteins targeted for proteasomes) from the cisternal space of the endoplasmic reticulum to the cytosol. ER-proteins are degraded in the cytosol by the 26S proteasome, a process known as Endoplasmic-reticulum-associated protein degradation, and therefore have to be transported by an appropriate channel. This retrotranslocon is still enigmatic.
It was initially believed that the Sec61 channel is responsible for this retrograde transport, implying that transport through Sec61 is not always unidirectional but also can be bidirectional.[13] However, the structure of Sec61 does not support this view and several different proteins have been suggested to be responsible for transport from the ER lumen into the cytosol.[14]
See also
References
- Johnson, A.E.; van Waes, M.A. (1999). "The translocon: a dynamic gateway at the ER membrane". Annu. Rev. Cell Dev. Biol. 15: 799–842. doi:10.1146/annurev.cellbio.15.1.799. PMID 10611978.
- Gold VA, Duong F, Collinson I (2007). "Structure and function of the bacterial Sec translocon". Mol. Membr. Biol. 24 (5–6): 387–94. doi:10.1080/09687680701416570. PMID 17710643.
- Mueller CA, Broz P, Cornelis GR (June 2008). "The type III secretion system tip complex and translocon". Mol. Microbiol. 68 (5): 1085–95. doi:10.1111/j.1365-2958.2008.06237.x. PMID 18430138.
- Deshaies RJ, Sanders SL, Feldheim DA, Schekman R (1991). "Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex". Nature. 349 (6312): 806–8. Bibcode:1991Natur.349..806D. doi:10.1038/349806a0. PMID 2000150.
- Van Den Berg, B; Clemons Jr, W. M.; Collinson, I; Modis, Y; Hartmann, E; Harrison, S. C.; Rapoport, T. A. (January 2004). "X-ray structure of a protein-conducting channel". Nature. 427 (6969): 36–44. Bibcode:2004Natur.427...36B. doi:10.1038/nature02218. PMID 14661030.
- Breyton C, Haase W, Rapoport TA, Kühlbrandt W, Collinson I (August 2002). "Three-dimensional structure of the bacterial protein-translocation complex SecYEG". Nature. 418 (6898): 662–5. Bibcode:2002Natur.418..662B. doi:10.1038/nature00827. PMID 12167867.
- Pfeffer S, Dudek J, Gogala M, Schorr S, Linxweiler J, Lang S, Becker T, Beckmann R, Zimmermann R, Förster F (2014). "Structure of the mammalian oligosaccharyl-transferase complex in the native ER protein translocon". Nat. Commun. 5 (5): 3072. Bibcode:2014NatCo...5E3072P. doi:10.1038/ncomms4072. PMID 24407213.
- Osborne, AR; Rapoport, TA; van den Berg, B (2005). "Protein translocation by the Sec61/SecY channel". Annual Review of Cell and Developmental Biology. 21: 529–50. doi:10.1146/annurev.cellbio.21.012704.133214. PMID 16212506.
- Simon, Sanford M.; Blobel, Günter (1991). "A protein-conducting channel in the endoplasmic reticulum". Cell. 65 (3): 371–380. doi:10.1016/0092-8674(91)90455-8.
- Simon, Sanford M.; Blobel, Günter (1992). "Signal peptides open protein-conducting channels in E. coli". Cell. 69 (4): 677–684. doi:10.1016/0092-8674(92)90231-z.
- Zhu, L.; Kaback, H.R.; Dalbey, R.E. (2013). "YidC protein, a molecular chaperone for LacY protein folding via the SecYEG protein machinery". The Journal of Biological Chemistry. 288 (39): 28180–28194. doi:10.1074/jbc.M113.491613. PMC 3784728. PMID 23928306.
- Lycklama A Nijeholt, J.A.; Driessen, A.J. (2012). "The bacterial Sec-translocase: structure and mechanism". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 367 (1592): 1016–1028. doi:10.1098/rstb.2011.0201. PMC 3297432. PMID 22411975.
- Römisch K (December 1999). "Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane". J. Cell Sci. 112 (23): 4185–91. PMID 10564637.
- Hampton, R. Y.; Sommer, T. (2012). "Finding the will and the way of ERAD substrate retrotranslocation". Current Opinion in Cell Biology. 24 (4): 460–6. doi:10.1016/j.ceb.2012.05.010. PMID 22854296.