Picloram
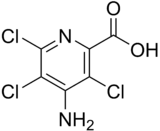
Picloram is a systemic herbicide used for general woody plant control. It also controls a wide range of broad-leaved weeds, but most grasses are resistant.[3] A chlorinated derivative of picolinic acid, picloram is in the pyridine family of herbicides.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-Amino-3,5,6-trichloro-2-pyridinecarboxylic acid | |
| Other names
Picloram Tordon Grazon | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | ATCP |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.016.034 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H3Cl3N2O2 | |
| Molar mass | 241.45 g·mol−1 |
| Appearance | colorless to white crystalline solid[1] |
| Odor | chlorine-like[1] |
| Melting point | 218.5 °C (425.3 °F; 491.6 K) decomposes |
| 0.04% (20°C)[1] 430 mg/L at 25 deg C[2] | |
| Vapor pressure | 0.0000006 mmHg (35°C)[1] |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
REL (Recommended) |
none established[1] |
IDLH (Immediate danger) |
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Picloram can be sprayed on foliage, injected into plants, applied to cut surfaces, or placed at the base of the plant where it will leach to the roots. Once absorbed by the foliage, stem, or roots, picloram is transported throughout the plant.
Herbicides containing Picloram are sold under a variety of brand names. Dow Chemicals and now Dow AgroSciences sell herbicides containing it under the brand name Tordon.[4]
During the Vietnam War, a mixture of picloram and other herbicides were combined to make Agent White (commercially available as Tordon 101) and enhanced Agent Orange which was previously conducted by the British military during the Malayan Emergency. Large quantities of these herbicides were sprayed by U.S. forces in areas where they considered its long-term persistence desirable, such as inland forests.[5]
Safety
Picloram is of moderate toxicity to the eyes and only mildly toxic on the skin.[3] There is no documented history of human intoxication by picloram so symptoms of acute exposure are difficult to characterize.
Picloram is the most persistent of its family of herbicides.[6] It does not adhere to soil and so may leach to groundwater, and has in fact been detected there. It is degraded in soil and water mainly by microbes. Picloram has very little tendency to accumulate in aquatic life.
Gardeners who use dung as fertilizer should check to make certain that the animal source has not grazed on picloram-treated hay, as the dung still has broadleaf-killing potency.[7]
In regards to occupational exposures, the Occupational Safety and Health Administration has established a permissible exposure limit of 15 mg/m3 total exposure and 5 mg/m3 for respiratory exposure, over an eight-hour workshift.[8]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0514". National Institute for Occupational Safety and Health (NIOSH).
- "Picloram".
- Picloram Pesticide Information Profile, Pesticide Management Education Program, Cornell University.
- Stanley A. Greene (2005). Sittig's Handbook of Pesticides and Agricultural Chemicals. William Andrew. p. 717. ISBN 978-0-8155-1903-4.
- Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides; Institute of Medicine (1994). Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. National Academies Press. pp. 89–90. ISBN 978-0-309-55619-4.
- Consumer Factsheet on: PICLORAM, U.S. Environmental Protection Agency
- Use Caution When Harvesting and Feeding Ditch Hay Archived 2010-06-14 at the Wayback Machine, U. Minnesota Extension
- NIOSH Pocket Guide to Chemical Hazards, Centers for Disease Control and Prevention
External links
- Picloram in the Pesticide Properties DataBase (PPDB)