tert-Butyllithium
tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon acids, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.[1]
 | |
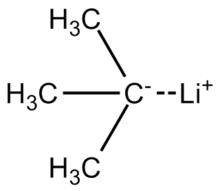 | |
| Names | |
|---|---|
| Preferred IUPAC name
tert-Butyllithium | |
| Identifiers | |
3D model (JSmol) |
|
| 3587204 | |
| ChemSpider | |
| ECHA InfoCard | 100.008.939 |
| EC Number |
|
PubChem CID |
|
| UN number | 3394 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiC 4H 9 | |
| Molar mass | 64.055 g mol−1 |
| Appearance | Colorless solid |
| Density | 660 mg cm−3 |
| Boiling point | 36 to 40 °C (97 to 104 °F; 309 to 313 K) |
| Reacts | |
| Acidity (pKa) | 45–53 |
| Hazards | |
| GHS pictograms |      |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H250, H260, H300, H304, H310, H314, H330, H336, H411 |
| P210, P222, P223, P231+232, P370+378, P422 | |
| NFPA 704 (fire diamond) | |
| Flash point | −6.6 °C (20.1 °F; 266.5 K) |
| Related compounds | |
Related compounds |
n-Butyllithium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and bonding
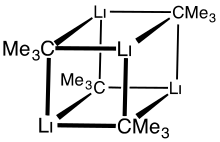
Like other organolithium compounds, tert-butyllithium is a cluster. Whereas n-butyllithium exists both as a hexamer and a tetramer, tert-Butyllithium exists as tetramer with a cubane structure. Bonding in organolithium clusters involves sigma delocalization and significant Li—Li bonding.[2]
The lithium–carbon bond in tert-butyllithium is highly polarized, having about 40 percent ionic character. The molecule reacts like a carbanion, as is represented by these two resonance structures.[3] (Given the polarity calculations on the C–Li bond, the "real" structure of a single molecule of t-butyllithium is likely a near-average of the two resonance contributors shown, in which the central carbon atom has a ~50% partial negative charge while the lithium atom has a ~50% partial positive charge.)
Chemical properties
Similar to n-butyllithium, tert-butyllithium can be used for the exchange of lithium with halogens and for the deprotonation of amines and activated C—H compounds.
This compound and other alkyllithium compounds are known to react with ether solvents; the half-life of tert-butyllithium is 60 minutes at 0 °C in diethyl ether, 40 minutes at −20 °C in tetrahydrofuran (THF),[4] and about 11 minutes at −70 °C in dimethoxyethane.[5] In this example, the reaction of tert-butyllithium with (THF) is shown:
To minimize degradation by these solvents, reactions involving tert-butyllithium are often conducted at very low temperatures in special solvents, such as the Trapp solvent mixture.
Safety
tert-Butyllithium is a pyrophoric substance, meaning that it easily catches fire on exposure to air. (A precise definition of a pyrophoric material is one "that ignite[s] spontaneously in air at or below 54.55 °C (130.19 °F)".[6]) The solvents used in common commercial preparations are themselves flammable. While it is possible to work with this compound using cannula transfer, traces of tert-butyllithium at the tip of the needle or cannula may catch fire and clog the cannula with lithium salts. While some researchers take this "pilot light" effect as a sign that the product is "fresh" and has not degraded due to time or improper storage/handling, others prefer to enclose the needle tip or cannula in a short glass tube, which is flushed with an inert gas and sealed at each end with septa.[7] Serious laboratory accidents involving tert-butyllithium have occurred. For example, in 2008 a staff research assistant, Sheharbano Sangji, in the lab of Patrick Harran[8] at the University of California, Los Angeles, died after being severely burned by a fire ignited by tert-butyllithium.[9][10][11]
Large-scale reactions may lead to runaway reactions, fires, and explosions when tert-butyllithium is mixed with ethers such as diethyl ether, and tetrahydrofuran. The use of hydrocarbon solvents may be preferred.
Air-free techniques are important so as to prevent this compound from reacting violently with oxygen and moisture in the air:
- t-BuLi + O2 → t-BuOOLi
- t-BuLi + H2O → t-BuH + LiOH
References
- Bartlett, Paul D.; C. Gardner Swain; Robert B. Woodward (1941). "t-Butyllithium". J. Am. Chem. Soc. 63 (11): 3229–3230. doi:10.1021/ja01856a501.
- Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2
- Organometallic reagents: sources of nucleophilic carbon for alcohol synthesis. K. P. C. Vollhardt, N. E. Schore: Organic Chemistry : Structure And Function. 3rd edition, 1999, §8.7.
- Stanetty, P; Koller, H.; Mihovilovic, M. (1992). "Directed ortho lithiation of phenylcarbamic acid 1,1-dimethylethyl ester (N-BOC-aniline). Revision and improvements". Journal of Organic Chemistry. 57 (25): 6833–6837. doi:10.1021/jo00051a030.
- Fitt, J. J.; Gschwend, H. E. (1984). "Reaction of n-, sec-, and tert-butyllithium with dimethoxyethane (DME): a correction". Journal of Organic Chemistry. 49: 209–210. doi:10.1021/jo00175a056.
- SEMI, standard F6-92, Guide for Secondary Containment of Hazardous Gas Piping Systems, as cited by ChemiCool.com
- Errington, R. M. (1997). Advanced practical inorganic and metalorganic chemistry (Google Books excerpt). London: Blackie Academic & Professional. pp. 47–48. ISBN 978-0-7514-0225-4.
- "Harran Lab: UCLA".
- Jyllian Kemsley (2009-01-22). "Researcher Dies After Lab Fire". Chemical & Engineering News.
- Jyllian Kemsley (2009-04-03). "Learning From UCLA: Details of the experiment that led to a researcher's death prompt evaluations of academic safety practices". Chemical & Engineering News.
- Los Angeles Times, 2009-03-01



