Sulfonylurea
Sulfonylureas (UK: sulphonylurea) are a class of organic compounds used in medicine and agriculture, for example as antidiabetic drugs widely used in the management of diabetes mellitus type 2. They act by increasing insulin release from the beta cells in the pancreas.[1] A number of sulfonylureas are also used as herbicides, because they can interfere with plant biosynthesis of certain amino acids.[2] Sulfonylureas are also used experimentally to inhibit interleukin 1 beta release from the NALP3 (or NLRP3) inflammasome.[3]
Drugs
- First-generation drugs include acetohexamide, carbutamide, chlorpropamide, glycyclamide (tolcyclamide), metahexamide, tolazamide and tolbutamide.
- Second-generation drugs include glibenclamide (glyburide), glibornuride, gliclazide,[4] glipizide, gliquidone, glisoxepide and glyclopyramide.
- Third-generation drugs include glimepiride, although it is sometimes considered a second-generation agent.[5][6]
Medical uses
Sulfonylureas are used primarily for the treatment of diabetes mellitus type 2. Sulfonylureas are ineffective where there is absolute deficiency of insulin production such as in type 1 diabetes or post-pancreatectomy.
Sulfonylureas can be used to treat some types of neonatal diabetes. While historically, people with hyperglycemia and low blood insulin levels were diagnosed with type 1 diabetes by default, it has been found that patients who receive this diagnosis before 6 months of age are often, in fact, candidates for receiving sulfonylureas rather than insulin throughout life.[7]
While prior sulfonylureas were associated with worse outcomes, newer agents do not appear to increase the risk of death, heart attacks, or strokes.[8]
Side effects
Sulfonylureas – as opposed to metformin, the thiazolidinediones, exenatide, pramlintide and other newer treatments – agents may induce hypoglycemia as a result of excesses in insulin production and release. This typically occurs if the dose is too high, and the patient is fasting. Some people attempt to change eating habits to prevent this, however it can be counter productive.
Like insulin, sulfonylureas can induce weight gain, mainly as a result of their effect to increase insulin levels and thus utilization of glucose and other metabolic fuels. Other side-effects are: gastrointestinal upset, headache and hypersensitivity reactions.
The safety of sulfonylurea therapy in pregnancy is unestablished. Prolonged hypoglycemia (4 to 10 days) has been reported in children borne to mothers taking sulfonylureas at the time of delivery.[9] Impairment of liver or kidney function increase the risk of hypoglycemia, and are contraindications. Since other antidiabetic drugs cannot be used either under these circumstances, insulin therapy is typically recommended during pregnancy and in liver and kidney failure, although some of the newer agents offer potentially better options.
A 2014 Cochrane review found tentative evidence that people treated with sulfonylureas have fewer non-fatal cardiovascular events than those treated with metformin (RR 0.7) but a higher risk of severe hypoglycemia (RR 5.6). There was not enough data available to determine the risk of mortality or of cardiovascular mortality.[10] An earlier review by the same group found a statistically significant increase in the risk of cardiovascular death for first generation sulfonylureas relative to placebo (RR 2.6) but there was not enough data to determine the relative risk of first generation sulfonylureas relative to insulin (RR 1.4). Likewise it was not possible to determine the relative mortality risk of second generation sulfonylureas relative to metformin (RR 1.0), insulin (RR 1.0), or placebo.[11] The FDA requires sulfonylureas to carry a label warning regarding increased risk of cardiovascular death.[9]
Second-generation sulfonylureas have increased potency by weight, compared to first-generation sulfonylureas. Similarly, ACCORD (Action to Control Cardiovascular Risk in Diabetes)[12] and the VADT (Veterans Affairs Diabetes Trial)[13] studies showed no reduction in heart attack or death in patients assigned to tight glucose control with various drugs.
Interactions
Drugs that potentiate or prolong the effects of sulfonylureas and therefore increase the risk of hypoglycemia include acetylsalicylic acid and derivatives, allopurinol, sulfonamides, and fibrates. Drugs that worsen glucose tolerance, contravening the effects of antidiabetics, include corticosteroids, isoniazid, oral contraceptives and other estrogens, sympathomimetics, and thyroid hormones. Sulfonylureas tend to interact with a wide variety of other drugs, but these interactions, as well as their clinical significance, vary from substance to substance.[14][15]
Structure
All pharmacological sulfonylureas contain a central S-arylsulfonylurea structure with a p-substituent on the phenyl ring (R1) and various groups terminating the urea N′ end group (R2). Chemically, this functionality can be easily installed by reacting aryl sulfonamides (R1—C6H4—SO2NH2) with isocyanates (R2—NCO).
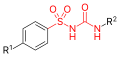 Sulfonylurea group highlighted in (red)
Sulfonylurea group highlighted in (red)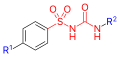 General formula of a sulfonylurea, showing the sulfonylurea backbone itself in red and the side chains that distinguish each compound in blue.
General formula of a sulfonylurea, showing the sulfonylurea backbone itself in red and the side chains that distinguish each compound in blue. Chlorpropamide (1st generation)
Chlorpropamide (1st generation) Tolazamide (1st generation)
Tolazamide (1st generation) Gliclazide (2nd generation)
Gliclazide (2nd generation) Glimepiride (2nd generation)
Glimepiride (2nd generation)
Mechanism of action
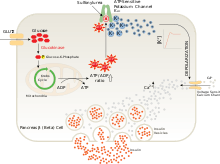
Sulfonylureas bind to and close ATP-sensitive K+ (KATP) channels on the cell membrane of pancreatic beta cells, which depolarizes the cell by preventing potassium from exiting. This depolarization opens voltage-gated Ca2+ channels. The rise in intracellular calcium leads to increased fusion of insulin granulae with the cell membrane, and therefore increased secretion of mature insulin.[16]
There is some evidence that sulfonylureas also sensitize β-cells to glucose, that they limit glucose production in the liver, that they decrease lipolysis (breakdown and release of fatty acids by adipose tissue) and decrease clearance of insulin by the liver.
The KATP channel is an octameric complex of the inward-rectifier potassium ion channel Kir6.x and sulfonylurea receptor SUR which associate with a stoichiometry of 4:4.[16]
Furthermore, it has been shown that sulfonylureas interact with the nucleotide exchange factor Epac2.[17][18] Mice lacking this factor exhibited a decreased glucose-lowering effect upon sulfonylurea treatment.
History
Sulfonylureas were discovered, in 1942, by the chemist Marcel Janbon and co-workers,[19] who were studying sulfonamide antibiotics and discovered that the compound sulfonylurea induced hypoglycemia in animals.[20]
Herbicides
A large number of sulfonylureas are used as herbicides. They function by interfering with biosynthesis of the amino acids valine, isoleucine, and leucine, specifically via acetolactate synthase inhibition. Compounds in this class include amidosulfuron, azimsulfuron, bensulfuron-methyl, chlorimuron-ethyl, chlorsulfuron, ethametsulfuron-methyl, cinosulfuron, ethoxysulfuron, flazasulfuron, flupyrsulfuron-methyl-sodium, imazosulfuron, metsulfuron-methyl, nicosulfuron, oxasulfuron, primisulfuron-methyl, prosulfuron, pyrazosulfuron-ethyl, rimsulfuron, sulfometuron-methyl, sulfosulfuron, thifensulfuron-methyl, triasulfuron, tribenuron-methyl, and triflusulfuron-methyl.[21]
References
- Seino S (August 2012). "Cell signalling in insulin secretion: the molecular targets of ATP, cAMP and sulfonylurea". Diabetologia. 55 (8): 2096–108. doi:10.1007/s00125-012-2562-9. PMID 22555472.
- Duggleby RG, McCourt JA, Guddat LW (2008). "Structure and mechanism of inhibition of plant acetohydroxyacid synthase". Plant Physiology and Biochemistry : PPB. 46 (3): 309–24. doi:10.1016/j.plaphy.2007.12.004. PMID 18234503.
- Coll R (March 2015). "A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases". Nature Medicine. 21 (3): 248–255. doi:10.1038/nm.3806. PMC 4392179. PMID 25686105.
- Karmoker J, Priya R, Sarkar S, Islam S (2017). "Comparative in vitro equivalence evaluation of some local Gliclazide brands of Bangladesh" (PDF). The Pharma Innovation Journal. 6: 152–157. Retrieved 2017-05-15.
- Triplitt CL, Reasner CA (2011). "Chapter 83: diabetes mellitus". In DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM (eds.). Pharmacotherapy: a pathophysiologic approach (8th ed.). New York, NY: McGraw-Hill. p. 1274. ISBN 978-0-07-170354-3.
- Davidson J (2000). Clinical diabetes mellitus: a problem-oriented approach. Stuttgart: Thieme. p. 422. ISBN 978-0-86577-840-5.
- Greeley SA, Tucker SE, Naylor RN, Bell GI, Philipson LH (August 2010). "Neonatal diabetes mellitus: a model for personalized medicine". Trends in Endocrinology and Metabolism. 21 (8): 464–72. doi:10.1016/j.tem.2010.03.004. PMC 2914172. PMID 20434356.
- Rados DV, Pinto LC, Remonti LR, Leitão CB, Gross JL (June 2016). "Correction: The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials". PLoS Medicine. 13 (6): e1002091. doi:10.1371/journal.pmed.1002091. PMC 4920361. PMID 27340828.
- "www.accessdata.fda.gov" (PDF).
- Hemmingsen B, Schroll JB, Wetterslev J, Gluud C, Vaag A, Sonne DP, Lundstrøm LH, Almdal T (Jul 2014). "Sulfonylurea versus metformin monotherapy in patients with type 2 diabetes: a Cochrane systematic review and meta-analysis of randomized clinical trials and trial sequential analysis". CMAJ Open. 2 (3): E162–75. doi:10.9778/cmajo.20130073. PMC 4185978. PMID 25295236.
- Hemmingsen B, Schroll JB, Lund SS, Wetterslev J, Gluud C, Vaag A, Sonne DP, Lundstrøm LH, Almdal T (2013). "Sulphonylurea monotherapy for patients with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 4 (4): CD009008. doi:10.1002/14651858.CD009008.pub2. PMID 23633364. (Retracted, see doi:10.1002/14651858.cd009008.pub3. If this is an intentional citation to a retracted paper, please replace
{{Retracted}}with{{Retracted|intentional=yes}}.) - Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Probstfield JL, Simons-Morton DG, Friedewald WT (Jun 2008). "Effects of intensive glucose lowering in type 2 diabetes". The New England Journal of Medicine. 358 (24): 2545–59. doi:10.1056/NEJMoa0802743. PMC 4551392. PMID 18539917.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD (Jan 2009). "Glucose control and vascular complications in veterans with type 2 diabetes". The New England Journal of Medicine. 360 (2): 129–39. doi:10.1056/NEJMoa0808431. PMID 19092145.
- Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
- Dinnendahl V, Fricke U, eds. (2010). Arzneistoff-Profile (in German). 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- Proks P, Reimann F, Green N, Gribble F, Ashcroft F (2002). "Sulfonylurea stimulation of insulin secretion". Diabetes. 51 (Suppl 3): S368–76. doi:10.2337/diabetes.51.2007.S368. PMID 12475777.
- Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S (Jul 2009). "The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs". Science. 325 (5940): 607–10. doi:10.1126/science.1172256. PMID 19644119.
- Takahashi T, Shibasaki T, Takahashi H, Sugawara K, Ono A, Inoue N, Furuya T, Seino S (Oct 2013). "Antidiabetic sulfonylureas and cAMP cooperatively activate Epac2A". Science Signaling. 6 (298): ra94. doi:10.1126/scisignal.2004581. PMID 24150255.
- Janbon M, Chaptal J, Vedel A, Schaap J (1942). "Accidents hypoglycémiques graves par un sulfamidothiodiazol (le VK 57 ou 2254 RP)". Montpellier Med. 441: 21–22.
- Patlak M (Dec 2002). "New weapons to combat an ancient disease: treating diabetes". FASEB Journal. 16 (14): 1853. doi:10.1096/fj.02-0974bkt. PMID 12468446.
- Arnold P. Appleby, Franz Müller, Serge Carpy "Weed Control" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_165