Sulfonamide
In chemistry, the sulfonamide functional group (also spelled sulphonamide) is -S(=O)2-NH2, a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. The amine center is no longer basic. The S-N bond is cleaved only with difficulty. Because of the rigidity of the functional group, sulfonamides are typically crystalline. For this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.[1]
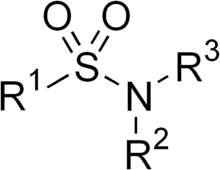
A sulfonamide (compound) is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2. Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or variation of sulfanilamide. The first sulfonamide was discovered in Germany in 1932.[2]
Synthesis
Sulfonamides can be prepared in the laboratory in many ways. The classic approach entails the reaction of sulfonyl chlorides with an amine.
- RSO2Cl + R2NH → RSO2NR2 + HCl
A base such as pyridine is typically added to absorb the HCl that is generated. Illustrative is the synthesis of sulfonylmethylamide.[3] A readily available sulfonyl chloride source is tosyl chloride.[4] The reaction of primary and secondary amines with benzenesulfonyl chloride is the basis of the Hinsberg reaction, a method for detecting primary and secondary amines.
Sultams
Sultams are cyclic sulfonamides. Bioactive sultams include the antiinflammatory ampiroxicam and the anticonvulsant sultiame. Sultams are prepared analogously to other sulfonamides, allowing for the fact that sulfonic acids are deprotonated by amines. They are often prepared by one-pot oxidation of disulfides or thiols linked to amines.[5] An alternative synthesis of sultams involves initial preparation of a linear sulfonamide, followed by intramolecular C-C bond formation (i.e. cyclization), a strategy that was used in the synthesis of a sultam-based deep-blue emitter for organic electronics.[6]
- Sulfonamide-based compounds
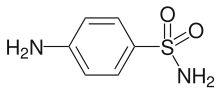 Sulfanilamide, a compound that foreshadowed the development of sulfa drugs
Sulfanilamide, a compound that foreshadowed the development of sulfa drugs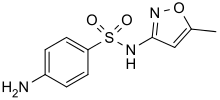 Sulfamethoxazole is a widely used antibiotic.
Sulfamethoxazole is a widely used antibiotic. Ampiroxicam is a sultam used as an antiinflammatory drug.
Ampiroxicam is a sultam used as an antiinflammatory drug.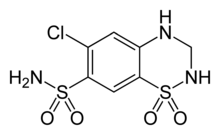 Hydrochlorothiazide is a drug that features both acyclic and cyclic sulfonamide groups.
Hydrochlorothiazide is a drug that features both acyclic and cyclic sulfonamide groups.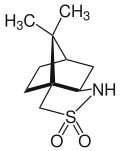 Camphorsultam is a sultam used as a chiral auxiliary in organic synthesis.
Camphorsultam is a sultam used as a chiral auxiliary in organic synthesis.
Sulfinamides
The related sulfinamides (R(S=O)NHR) are amides of sulfinic acids (R(S=O)OH) (see sulfinyl). Chiral sulfinamides such as tert-butanesulfinamide, p-toluenesulfinamide[7][8] and 2,4,6-trimethylbenzenesulfinamide[9] are relevant to asymmetric synthesis.
Disulfonimides
Bis(trifluoromethanesulfonyl)aniline is a source of the triflyl (CF3SO2+) group.
The disulfonimides are of the type R-S(=O)2-N(H)-S(=O)2-R' with two sulfonyl groups flanking an amine.[10] As with sulfinamides this class of compounds is used as catalysts in enantioselective synthesis.[10][11][12]
See also
References
- Actor, P.; Chow, A. W.; Dutko, F. J.; McKinlay, M. A. "Chemotherapeutics". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_173.CS1 maint: multiple names: authors list (link)
- Levy, Stuart B. (2002). The antibiotic paradox : how the misuse of antibiotics destroys their curative powers (2 ed.). Cambridge, Massachusetts: Perseus Publ. p. 51. ISBN 9780738204406.
- Organic Syntheses, Coll. Vol. 4, p.943 (1963); Vol. 34, p.96 (1954). Online Article
- Organic Syntheses, Coll. Vol. 5, p.39 (1973); Vol. 48, p.8 (1968) Online Article
- Rassadin, V.; Grosheva, D.; Tomashevskii, A. Sokolov, V. "Methods of Sultam Synthesis" Chemistry of Heterocyclic Compounds 2013, Vol. 49, p39-65. 27. doi:10.1007/s10593-013-1231-3.
- Virk, Tarunpreet Singh; Ilawe, Niranjan V.; Zhang, Guoxian; Yu, Craig P.; Wong, Bryan M.; Chan, Julian M. W. (2016). "Sultam-Based Hetero[5]helicene: Synthesis, Structure, and Crystallization-Induced Emission Enhancement". ACS Omega. 1: 1336–1342. doi:10.1021/acsomega.6b00335. PMC 6640820. PMID 31457199.
- Organic Syntheses, Coll. Vol. 10, p.47 (2004); Vol. 77, p.50 (2000). Link
- Org. Synth. 2007, 84, 129-138 Link
- Org. Synth. 2006, 83, 131-140 Link
- James, Thomas; van Gemmeren, Manuel; List, Benjamin (2015). "Development and Applications of Disulfonimides in Enantioselective Organocatalysis". Chem. Rev. 115: 9388–9409. doi:10.1021/acs.chemrev.5b00128.
- Treskow, M.; Neudörfl, J.; Giernoth, R. (2009). "BINBAM – A New Motif for Strong and Chiral Brønsted Acids". Eur. J. Org. Chem. 2009: 3693–3697. doi:10.1002/ejoc.200900548.
- García-García, P.; Lay, F.; García-García, P.; Rabalakos, C.; List, B. (2009). "A Powerful Chiral Counteranion Motif for Asymmetric Catalysis". Angew. Chem. Int. Ed. 48: 4363–4366. doi:10.1002/anie.200901768.