Sulfinamide
Sulfinamide is a functional group in organosulfur chemistry with the structural formula RS(O)NR'2 (where R and R' are organic substituents). This functionality is composed of a sulfur-carbon (S-C) and sulfur-nitrogen (S-N) single bonds, as well as a sulfur-oxygen double bond (S=O), resulting in a tetravalent sulfur centre (in equilibrium with its trivalent zwitterionic form). As a non-bonding electron pair is also present on the sulfur, these compounds are also chiral, and are referred to as S-chiral sulfinamides. Sulfinamides are amides of sulfinic acid (RS(O)OH).
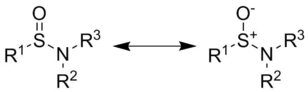
As sulfinamides do not undergo inversion, their chirality remains conserved and they can therefore be synthesised and/or isolated in enantiopure forms. This has led to their use as chiral ammonia equivalents and more broadly as chiral auxiliaries. The most commonly sulfinamides for these synthetic applications are tert-butanesulfinamide (Ellman's sulfinamide), p-toluenesulfinamide (Davis' sulfinamide), and 2,4,6-trimethylbenzenesulfinamide.[2][3][4]
Sulfinamides arise in nature by the addition of nitroxyl (HNO) to thiols:[5]
- RSH + HNO → RS(O)NH2
References
- Eccles, K. S.; Morrison, R. E.; Daly, C. A.; O'Mahony, G. E.; Maguire, A. R.; Lawrence, S. E. (2013). "Co-crystallisation Through Halogen Bonding with Racemic or Enantiopure Sulfinamides". CrystEngComm. 15: 7571–7575. doi:10.1039/C3CE40932E.
- Fanelli, D. L.; Szewczyk, J. M.; Zhang, Y.; Reddy, G. V.; Burns, D. M.; Davis, F. A. (2000). "SULFINIMINES (THIOOXIMINE S-OXIDES): ASYMMETRIC SYNTHESIS OF METHYL (R)-(+)-β-PHENYLALANATE FROM (S)-(+)-N-(BENZYLIDENE)-p-TOLUENESULFINAMIDE". Organic Syntheses. 77: 50.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 47
- Ruano, J. L.; Alemán, J.; Parra, A.; Cid, M. B. (2007). "PREPARATION OF N-p-TOLYLSULFONYL-(E)-1-PHENYLETHYLIDENEIMINE". Organic Syntheses. 84: 129.CS1 maint: multiple names: authors list (link)
- Ramachandar, T.; Wu, Y.; Zhang, J.; Franklin A. Davis (2006). "(S)-(+)-2,4,6-TRIMETHYLBENZENESULFINAMIDE". Organic Syntheses. 83: 131.CS1 maint: multiple names: authors list (link)
- Keceli, Gizem; Toscano, John P. (2014-06-10). "Reactivity of C-Terminal Cysteines with HNO". Biochemistry. 53 (22): 3689–3698. doi:10.1021/bi500360x. ISSN 0006-2960.