Sulfene
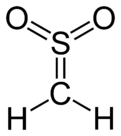
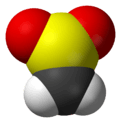
Sulfene is an extremely reactive chemical compound with the formula H2C=SO2. It is the simplest member of the sulfenes, the group of compounds which are S,S-dioxides of thioaldehydes and thioketones, and have the general formula R2C=SO2.[1][2][3]
| |||
| Names | |||
|---|---|---|---|
| Other names
Thioformaldehyde-S,S-dioxide; Methanethione dioxide | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH 2SO 2 | |||
| Molar mass | 78.090 g mol−1 | ||
| Structure | |||
| trigonal planar at C and S | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
The first general method for preparation of sulfene as an intermediate, reported simultaneously in 1962 by Gilbert Stork[4] and by Günther Optiz,[5] involved removal of hydrogen chloride from methanesulfonyl chloride using triethylamine in the presence of an enamine as trapping agent. The formation of a thietane 1,1-dioxide derivative was taken as evidence for the intermediacy of sulfene. Because of the highly electrophilic character of sulfene, the use of amines presents difficulties, since they can intercept the sulfene to form adducts. A simple alternative which avoids the use of amines involves desilylation of trimethylsilylmethanesulfonyl chloride with cesium fluoride in the presence of trapping agents.[6]
- (CH3)3SiCH2SO2Cl + CsF → [CH2=SO2] + (CH3)3SiF + CsCl
Alternatively, sulfenes can be stabilized by installing amido substituents on the alkylidene substituent. The extreme case is thiourea dioxide, which features planar amido groups.
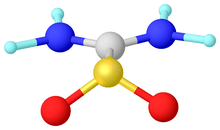 Structure of thioureadioxide ((H2N)2CSO2). Selected distances and angles: rS=O = 1.49, rS=C = 1.85.1, rC-N = 1.31 Å, sum of angles around S = 112°.[7]
Structure of thioureadioxide ((H2N)2CSO2). Selected distances and angles: rS=O = 1.49, rS=C = 1.85.1, rC-N = 1.31 Å, sum of angles around S = 112°.[7]
Reactions
Sulfenes react with enamines, ynamines, and 1,3-cyclopentadienes to give thietanes, thietes and Diels-Alder adducts, respectively. In the presence of a chiral tertiary amine complex, several sulfenes could be trapped with trichloroacetaldehyde (chloral) in a catalytic asymmetric synthesis of β-sulfones (four-membered ring sulfonate esters).[8] Sulfene can also undergo insertion into metal–hydrogen bonds.[9]
See also
- Sulfine - related functional group with the formula H2C=S=O
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "sulfenes". doi:10.1351/goldbook.S06095
- Zwanenburg, B (2004). "S,S-Dioxides of Thioaldehydes and Thioketones (Sulfenes and Derivatives)". Sci. Synth. 27: 123–134.
- King, JF (1975). "Return of Sulfenes". Acc. Chem. Res. 8 (1): 10–17. doi:10.1021/ar50085a002.
- Stork, G; Borowitz, IJ (1962). "Four-membered Sulfones from Enamines and Aliphatic Sulfonyl Halides". J. Am. Chem. Soc. 84 (2): 313. doi:10.1021/ja00861a042.
- Opitz, G; Adolph, H (1962). "Cycloaddition of Sulfenes to Enamines". Angew. Chem. Int. Ed. 1 (2): 113–114. doi:10.1002/anie.196201133.
- Block, E; Aslam, M (1982). "A New Sulfene Synthesis". Tetrahedron Lett. 23 (41): 4203–4206. doi:10.1016/S0040-4039(00)88704-3.
- R. A. L. Sullivan, A. Hargreaves (1962). "The Crystal and Molecular Structure of Thiourea Dioxide". Acta Crystallogr. 15: 675–682. doi:10.1107/S0365110X62001851.CS1 maint: uses authors parameter (link)
- Koch, FM; Peters, R (2011). "Lewis Acid/Base Catalyzed [2+2]-Cycloaddition of Sulfenes and Aldehydes: A Versatile Entry to Chiral Sulfonyl and Sulfinyl Derivatives". Chem. Eur. J. 17: 3679–3692. doi:10.1002/chem.201003542.
- Ingo-Peter Lorenz (April 1978). "Demonstration of "Sulfene" Insertion into the Metal–Hydrogen Bond". Angew. Chem. Int. Ed. 17 (4): 285–286. doi:10.1002/anie.197802851.