Appel reaction
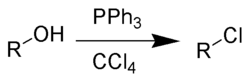
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride.[1] The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using carbon tetraiodide, methyl iodide or iodine gives alkyl iodides. The reaction is credited to and named after Rolf Appel,[2] it had however been described earlier.[3] The use of this reaction is becoming less common, due to carbon tetrachloride being restricted under the Montreal protocol.
| Appel reaction | |
|---|---|
| Named after | Rolf Appel |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | appel-reaction |
| RSC ontology ID | RXNO:0000406 |
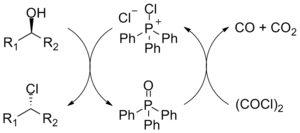
Drawbacks to the reaction are the use of toxic halogenating agents and the coproduction of organophosphorus product that must be separated from the organic product.[4] The phosphorus reagent can be used in catalytic quantities.[5][6] The corresponding alkyl bromide can also be synthesised by addition of lithium bromide as a source of bromide ions.
Mechanism
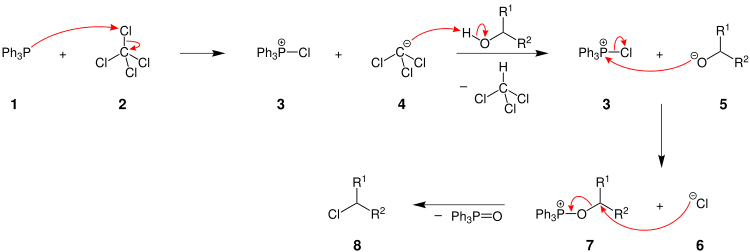
The Appel reaction begins with the formation of the phosphonium salt 3, which is thought to exist as a tight ion pair with 4[7] and therefore is unable to undergo an alpha-elimination to give dichlorocarbene. Deprotonation of the alcohol, forming chloroform, yields an alkoxide 5. The nucleophilic displacement of the chloride by the alkoxide yields intermediate 7. With primary and secondary alcohols, the halide reacts in a SN2 process forming the alkyl halide 8 and triphenylphosphine oxide. Tertiary alcohols form the products 6 and 7 via a SN1 mechanism.
The driving force behind this and similar reactions is the formation of the strong PO double bond.[8] The reaction is somewhat similar to the Mitsunobu Reaction, where the combination of an organophosphine as an oxide acceptor, an azo compound as a hydrogen acceptor reagent, and a nucleophile are used to convert alcohols to esters and other applications like this.[9]
Illustrative use of the Appel reaction is the chlorination of geraniol to geranyl chloride.[10]
Modifications
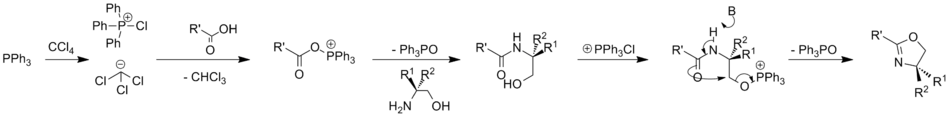
The Appel reaction is also effective on carboxylic acids; this has been used to convert them to oxazolines, oxazines and thiazolines.[11]
References
- Rolf Appel (1975). "Tertiary Phosphane/Tetrachloromethane, a Versatile Reagent for Chlorination, Dehydration, and P-N Linkage". Angewandte Chemie International Edition in English. 14 (12): 801–811. doi:10.1002/anie.197508011.
- http://www.chemie.uni-bonn.de/oc/geschichte
- Downie, I; Holmes, J; Lee, J (1966). "Preparation of Alkyl Chlorides Under Mild Conditions". Chemistry and Industry (22): 900. ISSN 0009-3068.
- Cadogan, J, ed. (1979). Organophosphorus Reagents in Organic Synthesis. London: Academic Press. ISBN 978-0-12-154350-1.
- Denton, Ross; An, Jie; Adeniran, Beatrice; Blake, Alexander; Lewis, William; Poulton, Andrew (2011). "Catalytic Phosphorus(V)-Mediated Nucleophilic Substitution Reactions: Development of a Catalytic Appel Reaction". Journal of Organic Chemistry. 76 (16): 6749–6767. doi:10.1021/jo201085r. PMID 21744876.
- van Kalkeren, Henri A.; Leenders, Stefan H. A. M.; Hommersom, C. (Rianne) A.; Rutjes, Floris P. J. T.; van Delft, Floris L. (2011). "In Situ Phosphine Oxide Reduction: A Catalytic Appel Reaction". Chemistry: A European Journal. 17 (40): 11290–11295. doi:10.1002/chem.201101563. PMID 21882274.
- Wang, Zerong (2009). "22: Appel Reaction". Comprehensive organic name reactions and reagents. Hoboken, N.J.: John Wiley. pp. 95–99. doi:10.1002/9780470638859.conrr022. ISBN 9780470638859.
- "Archived copy" (PDF). Archived from the original (PDF) on 2012-07-22. Retrieved 2012-07-11.CS1 maint: archived copy as title (link)
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Jose G. Calzada and John Hooz. "Geranyl chloride". Organic Syntheses.; Collective Volume, 6, p. 634
- Vorbrüggen, Helmut; Krolikiewicz, Konrad (January 1993). "A simple synthesis of Δ2-oxazines, Δ2-oxazines, Δ2-thiazolines and 2-substituted benzoxazoles". Tetrahedron. 49 (41): 9353–9372. doi:10.1016/0040-4020(93)80021-K.