Idoxuridine
Idoxuridine is an anti-herpesvirus antiviral drug.
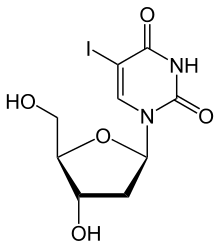 | |
| Clinical data | |
|---|---|
| Other names | Iododeoxyuridine; IUdR |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601062 |
| Pregnancy category |
|
| Routes of administration | topically |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.189 |
| Chemical and physical data | |
| Formula | C9H11IN2O5 |
| Molar mass | 354.100 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It is a nucleoside analogue, a modified form of deoxyuridine, similar enough to be incorporated into viral DNA replication, but the iodine atom added to the uracil component blocks base pairing. It is used only topically due to cardiotoxicity. It was synthesized by William Prusoff in the late 1950s.[1] Initially developed as an anticancer drug, idoxuridine became the first antiviral agent in 1962.[2]
Clinical use
Idoxuridine is mainly used topically to treat herpes simplex keratitis.[3] Epithelial lesions, especially initial attacks presenting with a dendritic ulcer, are most responsive to therapy, while infection with stromal involvement are less responsive.[4] Idoxuridine is ineffective against herpes simplex virus type 2 and varicella-zoster.[3]
Side effects
Common side effects of the eye drops include irritation, blurred vision and photophobia.[5] Corneal clouding and damage of the corneal epithelium may also occur.
Formulations and dosage
Idoxuridine is available as either a 0.5% ophthalmic ointment or as a 0.1% ophthalmic solution.[3] The dosage of the ointment is every 4 hours during day and once before bedtime.[3] The dosage of the solution is 1 drop in the conjunctival sac hourly during the day and every 2 hours during the night until definitive improvement, then 1 drop every 2 hours during the day and every 4 hours during the night.[3] Therapy is continued for 3–4 days after healing is complete, as demonstrated by fluorescein staining.[3]
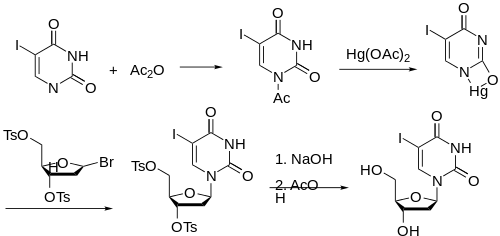
Synthesis
See also
References
- Prusoff, W. H. (1959). "Synthesis and biological activities of iododeoxyuridine, an analog of thymidine". Biochimica et Biophysica Acta. 32 (1): 295–6. doi:10.1016/0006-3002(59)90597-9. PMID 13628760.
- Wilhelmus KR (2015). "Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis". Cochrane Database Syst Rev. 1: CD002898. doi:10.1002/14651858.CD002898.pub5. PMC 4443501. PMID 25879115.
- Goodman and Gilman's The Pharmacological Basis of Therapeutics. Edited by Gilman AG, Rall TW, Nies AS, Taylor P. McGraw-Hill. 8th ed. 1990.
- Maxwell, E (1963). "Treatment of herpes keratitis with 5-iodo-2-deoxyuridine (IDU): a clinical evaluation of 1500 cases". Am. J. Ophthalmol. 56: 571–573. doi:10.1016/0002-9394(63)90006-0. PMID 14070708.
- Drugs.com: Idoxuridine ophthalmic
- Cheong, L.; Rich, M. A.; Eidinoff, M. L. (1960). "Introduction of the 5-Halogenated Uracil Moiety into Deoxyribonucleic Acid of Mammalian Cells in Culture". J. Biol. Chem. 235: 1441–1447. PMID 13809628.
- Chang, P. K.; Welch, A. D. (1963). "Iodination of 2'-Deoxycytidine and Related Substances1". Journal of Medicinal Chemistry. 6 (4): 428–430. doi:10.1021/jm00340a019. PMID 14184899.
Further reading
- Seth A, Misra A, Umrigar D (2004). "Topical liposomal gel of idoxuridine for the treatment of herpes simplex: pharmaceutical and clinical implications". Pharm Dev Technol. 9 (3): 277–289. doi:10.1081/PDT-200031432. PMID 15458233.
- Otto S (1998). "Radiopharmaceuticals (Strontium 89) and radiosensitizers (idoxuridine)". J Intraven Nurs. 21 (6): 335–7. PMID 10392098.
- Fauth E, Zankl H (1999). "Comparison of spontaneous and idoxuridine-induced micronuclei by chromosome painting". Mutat Res. 440 (2): 147–56. doi:10.1016/s1383-5718(99)00021-2. PMID 10209337.