Square antiprismatic molecular geometry
In chemistry, the square antiprismatic molecular geometry describes the shape of compounds where eight atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a square antiprism.[1] This shape has D4d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the dodecahedron and the bicapped trigonal prism.[2][3]
| Square antiprismatic molecular geometry | |
|---|---|
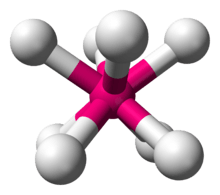 | |
| Examples | XeF2− 8, ReF− 8 |
| Point group | D4d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
Like with other high coordination numbers, eight-coordinate compounds are often distorted from idealized geometries, as illustrated by the structure of Na3TaF8. In this case, with the small Na+ ions, lattice forces are strong. With the diatomic cation NO+, the lattice forces are weaker, such as in (NO)2XeF8, which crystallizes with a more idealized square antiprismatic geometry.
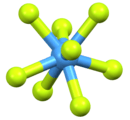 The distorted square antiprismatic [TaF8]3− anion in the Na3TaF8 lattice.[4]
The distorted square antiprismatic [TaF8]3− anion in the Na3TaF8 lattice.[4]-3D-balls-A.png) The square antiprismatic [XeF8]2− anion in the lattice of nitrosonium octafluoroxenate(VI), (NO)2XeF8.[5]
The square antiprismatic [XeF8]2− anion in the lattice of nitrosonium octafluoroxenate(VI), (NO)2XeF8.[5] Structure of the Bi82+ cluster in the [Bi8](GaCl4)2.
Structure of the Bi82+ cluster in the [Bi8](GaCl4)2.
Examples
- XeF2−
8 - IF−
8 - ReF−
8
References
- D. L. Kepert (1978). "Aspects of the Stereochemistry of Eight-Coordination". Progress in Inorganic Chemistry. 24: 179–249. doi:10.1002/9780470166253.ch4.
- Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi:10.1021/ic50187a041.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Langer, V.; Smrčok, L.; Boča, M. (2010). "Redetermination of Na3TaF8". Acta Crystallographica Section C. 66: pi85–pi86. doi:10.1107/S0108270110030556.CS1 maint: uses authors parameter (link)
- Peterson, W.; Holloway, H.; Coyle, A.; Williams, M. (Sep 1971). "Antiprismatic Coordination about Xenon: the Structure of Nitrosonium Octafluoroxenate(VI)". Science. 173 (4003): 1238–1239. Bibcode:1971Sci...173.1238P. doi:10.1126/science.173.4003.1238. ISSN 0036-8075. PMID 17775218.