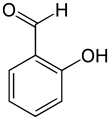
Salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula C6H4CHO-2-OH.[3] Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a key precursor to a variety chelating agents, some of which are commercially important.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxybenzaldehyde[1] | |||
| Other names
Salicylaldehyde Salicylic aldehyde o-Hydroxybenzaldehyde | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.783 | ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.123 g·mol−1 | ||
| Density | 1.146 g/cm3 | ||
| Melting point | −7 °C (19 °F; 266 K) | ||
| Boiling point | 196 to 197 °C (385 to 387 °F; 469 to 470 K) | ||
| -64.4·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | [2] | ||
| GHS pictograms |   | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H302, H315, H317, H319, H335, H411[2] | ||
| P280, P305+351+338[2] | |||
| Related compounds | |||
Related compounds |
Salicylic acid Benzaldehyde Salicylaldoxime | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
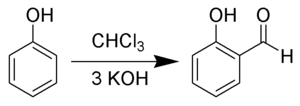
Salicylaldehyde is prepared from phenol and chloroform by heating with sodium hydroxide or potassium hydroxide in a Reimer–Tiemann reaction:[4]
Alternatively, it is produced by condensation of phenol or its derivatives with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde.
Salicylaldehydes in general may be prepared from the corresponding phenol by the Duff reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base.[5]
Natural occurrences
Salicylaldehyde was identified as a characteristic aroma component of buckwheat.[6]
It is also one of the components of castoreum, the exudate from the castor sacs of the mature North American beaver (Castor canadensis) and the European beaver (Castor fiber), used in perfumery.
Furthermore, salicylaldehyde occurs in the larval defensive secretions of several leaf beetle species that belong the subtribe Chrysomelina.[7] An example for a leaf beetle species that produces salicylaldehyde is the red poplar leaf beetle Chrysomela populi.
Reactions and applications
Salicylaldehyde is used to make the following:

- Oxidation with hydrogen peroxide gives catechol (1,2-dihydroxybenzene) (Dakin reaction).[8]
- Etherification with chloroacetic acid followed by cyclisation gives the heterocycle benzofuran (coumarone).[9] {The first step in this reaction to the substituted benzofuran is called the Rap–Stoermer condensation after E. Rap (1895) and R. Stoermer (1900).[10][11]
- Salicylaldehyde is converted to chelating ligands by condensation with amines. With ethylenediamine, it condenses to give the ligand salen. Hydroxylamine gives salicylaldoxime.
- Condensation with diethyl malonate gives 3-carbethoxycoumarin (a derivative of coumarin) by an aldol condensation.[12]
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Sigma-Aldrich Co., Salicylaldehyde. Retrieved on 2018-05-24.
- Merck Index, 11th Edition, 8295
- Brühne, F.; Wright, E. "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_463.CS1 maint: multiple names: authors list (link)
- Trond Vidar Hansen; Lars Skattebøl (2005). "Ortho-Formylation of Phenols; Preparation of 3-Bromosalicylaldehyde". Organic Syntheses. 82: 64.
- Janeš, D.; Kreft, S. (2008). "Salicylaldehyde is a characteristic aroma component of buckwheat groats". Food Chemistry. 109 (2): 293–298. doi:10.1016/j.foodchem.2007.12.032. PMID 26003350.
- Pauls, G., Becker, T., et al. (2016). Two Defensive Lines in Juvenile Leaf Beetles; Esters of 3-nitropropionic Acid in the Hemolymph and Aposematic Warning. Journal of Chemical Ecology 42 (3) 240-248.
- Dakin, H. D. (1923). "Catechol" (PDF). Organic Syntheses. 3: 28.; Collective Volume, 1, p. 149
- Burgstahler, A. W.; Worden, L. R. (1966). "Coumarone" (PDF). Organic Syntheses. 46: 28.CS1 maint: multiple names: authors list (link); Collective Volume, 5, p. 251
- Rap, E. (November 1895). "Sull' α-Benzoilcumarone" [On the α-Benzoylcoumaron]. Gazzetta Chimica Italiana. 2 (4): 285–290.
- Stoermer, R. (1900). "Synthesen und Abbaureactionen in der Cumaronreihe". Liebig's Annalen der Chemie. 312 (3): 237–336. doi:10.1002/jlac.19003120302.
- Horning, E. C.; Horning, M. G.; Dimmig, D. A. (1948). "3-Carbethoxycoumarin" (PDF). Organic Syntheses. 28: 24.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 165