RNA silencing
RNA silencing or RNA interference refers to a family of gene silencing effects by which gene expression is negatively regulated by non-coding RNAs such as microRNAs. RNA silencing may also be defined as sequence-specific regulation of gene expression triggered by double-stranded RNA (dsRNA).[1] RNA silencing mechanisms are highly conserved in most eukaryotes.[2] The most common and well-studied example is RNA interference (RNAi), in which endogenously expressed microRNA (miRNA) or exogenously derived small interfering RNA (siRNA) induces the degradation of complementary messenger RNA. Other classes of small RNA have been identified, including piwi-interacting RNA (piRNA) and its subspecies repeat associated small interfering RNA (rasiRNA).[3]
Background
RNA silencing describes several mechanistically related pathways which are involved in controlling and regulating gene expression.[4][5][6] RNA silencing pathways are associated with the regulatory activity of small non-coding RNAs (approximately 20–30 nucleotides in length) that function as factors involved in inactivating homologous sequences, promoting endonuclease activity, translational arrest, and/or chromatic or DNA modification.[7][8][9] In the context in which the phenomenon was first studied, small RNA was found to play an important role in defending plants against viruses. For example, these studies demonstrated that enzymes detect double-stranded RNA (dsRNA) not normally found in cells and digest it into small pieces that are not able to cause disease.[10][11][12][13][2]
While some functions of RNA silencing and its machinery are understood, many are not. For example, RNA silencing has been shown to be important in the regulation of development and in the control of transposition events.[14] RNA silencing has been shown to play a role in antiviral protection in plants as well as insects.[15] Also in yeast, RNA silencing has been shown to maintain heterochromatin structure.[16] However, the varied and nuanced role of RNA silencing in the regulation of gene expression remains an ongoing scientific inquiry. A range of diverse functions have been proposed for a growing number of characterized small RNA sequences—e.g., regulation of developmental, neuronal cell fate, cell death, proliferation, fat storage, haematopoietic cell fate, insulin secretion.[17]
RNA silencing functions by repressing translation or by cleaving messenger RNA (mRNA), depending on the amount of complementarity of base-pairing. RNA has been largely investigated within its role as an intermediary in the translation of genes into proteins.[18] More active regulatory functions, however, only began to be addressed by researchers beginning in the late-1990s.[19] The landmark study providing an understanding of the first identified mechanism was published in 1998 by Fire et al.,[1] demonstrating that double-stranded RNA could act as a trigger for gene silencing.[19] Since then, various other classes of RNA silencing have been identified and characterized.[4] Presently, the therapeutic potential of these discoveries is being explored, for example, in the context of targeted gene therapy.[20][21]
While RNA silencing is an evolving class of mechanisms, a common theme is the fundamental relationship between small RNAs and gene expression.[8] It has also been observed that the major RNA silencing pathways currently identified have mechanisms of action which may involve both post-transcriptional gene silencing (PTGS)[22] as well as chromatin-dependent gene silencing (CDGS) pathways.[4] CDGS involves the assembly of small RNA complexes on nascent transcripts and is regarded as encompassing mechanisms of action which implicate transcriptional gene silencing (TGS) and co-transcriptional gene silencing (CTGS) events.[23] This is significant at least because the evidence suggests that small RNAs play a role in the modulation of chromatin structure and TGS.[24][25]
Despite early focus in the literature on RNA interference (RNAi) as a core mechanism which occurs at the level of messenger RNA translation, others have since been identified in the broader family of conserved RNA silencing pathways acting at the DNA and chromatin level.[26] RNA silencing refers to the silencing activity of a range of small RNAs and is generally regarded as a broader category than RNAi. While the terms have sometimes been used interchangeably in the literature, RNAi is generally regarded as a branch of RNA silencing. To the extent it is useful to craft a distinction between these related concepts, RNA silencing may be thought of as referring to the broader scheme of small RNA related controls involved in gene expression and the protection of the genome against mobile repetitive DNA sequences, retroelements, and transposons to the extent that these can induce mutations.[27] The molecular mechanisms for RNA silencing were initially studied in plants[12] but have since broadened to cover a variety of subjects, from fungi to mammals, providing strong evidence that these pathways are highly conserved.[28]
At least three primary classes of small RNA have currently been identified, namely: small interfering RNA (siRNA), microRNA (miRNA), and piwi-interacting RNA (piRNA).
small interfering RNA (siRNA)
siRNAs act in the nucleus and the cytoplasm and are involved in RNAi as well as CDGS.[4] siRNAs come from long dsRNA precursors derived from a variety of single-stranded RNA (ssRNA) precursors, such as sense and antisense RNAs. siRNAs also come from hairpin RNAs derived from transcription of inverted repeat regions. siRNAs may also arise enzymatically from non-coding RNA precursors.[29] The volume of literature on siRNA within the framework of RNAi is extensive.
microRNA (miRNA)
The majority of miRNAs act in the cytoplasm and mediate mRNA degradation or translational arrest.[30] However, some plant miRNAs have been shown to act directly to promote DNA methylation.[31] miRNAs come from hairpin precursors generated by the RNaseIII enzymes Drosha and Dicer.[32] Both miRNA and siRNA form either the RNA-induced silencing complex (RISC) or the nuclear form of RISC known as RNA-induced transcriptional silencing complex (RITS).[33] The volume of literature on miRNA within the framework of RNAi is extensive.
Three prime untranslated regions and microRNAs
Three prime untranslated regions (3'UTRs) of messenger RNAs (mRNAs) often contain regulatory sequences that post-transcriptionally cause RNA interference. Such 3'-UTRs often contain both binding sites for microRNAs (miRNAs) as well as for regulatory proteins. By binding to specific sites within the 3'-UTR, miRNAs can decrease gene expression of various mRNAs by either inhibiting translation or directly causing degradation of the transcript. The 3'-UTR also may have silencer regions that bind repressor proteins that inhibit the expression of a mRNA.
The 3'-UTR often contains microRNA response elements (MREs). MREs are sequences to which miRNAs bind. These are prevalent motifs within 3'-UTRs. Among all regulatory motifs within the 3'-UTRs (e.g. including silencer regions), MREs make up about half of the motifs.
As of 2014, the miRBase web site,[34] an archive of miRNA sequences and annotations, listed 28,645 entries in 233 biologic species. Of these, 1,881 miRNAs were in annotated human miRNA loci. miRNAs were predicted to have an average of about four hundred target mRNAs (affecting expression of several hundred genes).[35] Freidman et al.[35] estimate that >45,000 miRNA target sites within human mRNA 3'UTRs are conserved above background levels, and >60% of human protein-coding genes have been under selective pressure to maintain pairing to miRNAs.
Direct experiments show that a single miRNA can reduce the stability of hundreds of unique mRNAs.[36] Other experiments show that a single miRNA may repress the production of hundreds of proteins, but that this repression often is relatively mild (less than 2-fold).[37][38]
The effects of miRNA dysregulation of gene expression seem to be important in cancer.[39] For instance, in gastrointestinal cancers, nine miRNAs have been identified as epigenetically altered and effective in down regulating DNA repair enzymes.[40]
The effects of miRNA dysregulation of gene expression also seem to be important in neuropsychiatric disorders, such as schizophrenia, bipolar disorder, major depression, Parkinson's disease, Alzheimer's disease and autism spectrum disorders.[41][42][43]
piwi-interacting RNA (piRNA)
piRNAs represent the largest class of small non-coding RNA molecules expressed in animal cells, deriving from a large variety of sources, including repetitive DNA and transposons.[44] However, the biogenesis of piRNAs is also the least well understood.[45] piRNAs appear to act both at the post-transcriptional and chromatin levels. They are distinct from miRNA due to at least an increase in terms of size and complexity. Repeat associated small interfering RNA (rasiRNAs) are considered to be a subspecies of piRNA.[3]
Mechanism
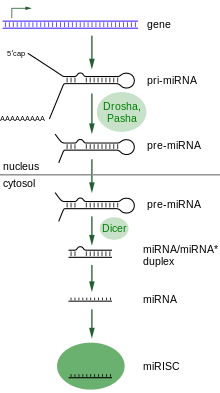
The most basic mechanistic flow for RNA Silencing is as follows: (For a more detailed explanation of the mechanism, refer to the RNAi:Cellular mechanism article.)
1: RNA with inverted repeats hairpin/panhandle constructs --> 2: dsRNA --> 3: miRNAs/siRNAs --> 4: RISC --> 5: Destruction of target mRNA
- It has been discovered that the best precursor to good RNA silencing is to have single stranded antisense RNA with inverted repeats which, in turn, build small hairpin RNA and panhandle constructs.[6] The hairpin or panhandle constructs exist so that the RNA can remain independent and not anneal with other RNA strands.
- These small hairpin RNAs and/or panhandles then get transported from the nucleus to the cytosol through the nuclear export receptor called exportin-5, and then get transformed into a dsRNA, a double stranded RNA, which, like DNA, is a double stranded series of nucleotides. If the mechanism didn't use dsRNAs, but only single strands, there would be a higher chance for it to hybridize to other "good" mRNAs. As a double strand, it can be kept on call for when it is needed.
- The dsRNA then gets cut up by a Dicer into small (21-28 nt = nucleotides long) strands of miRNAs (microRNAs) or siRNAs (short interfering RNAs.) A Dicer is an endoribonuclease RNase, which is a complex of a protein mixed with strand(s) of RNA.
- Lastly, the double stranded miRNAs/siRNAs separate into single strands; the antisense RNA strand of the two will combine with another endoribonuclease enzyme complex called RISC (RNA-induced silencing complex), which includes the catalytic component Argonaute, and will guide the RISC to break up the "perfectly complementary" target mRNA or viral genomic RNA so that it can be destroyed.[2][6]
- It means that based on a short sequence specific area, a corresponding mRNA will be cut. To make sure, it will be cut in many other places as well. (If the mechanism only worked with a long stretch, then there would be higher chance that it would not have time to match to its complementary long mRNA.) It has also been shown that the repeated-associated short interference RNAs (rasiRNA) have a role in guiding chromatin modification.[2]
For an animated explanation of the mechanism of RNAi by Nature Reviews, see the External Links section below.
Biological functions
Immunity against viruses or transposons
RNA silencing is the mechanism that our cells (and cells from all kingdoms) use to fight RNA viruses and transposons (which originate from our own cells as well as from other vehicles).[2] In the case of RNA viruses, these get destroyed immediately by the mechanism cited above. In the case of transposons, it's a little more indirect. Since transposons are located in different parts of the genome, the different transcriptions from the different promoters produce complementary mRNAs that can hybridize with each other. When this happens, the RNAi machinery goes into action, debilitating the mRNAs of the proteins that would be required to move the transposons themselves.[46]
Down-regulation of genes
For a detailed explanation of the down-regulation of genes, see RNAi:downregulation of genes
Up-regulation of genes
For a detailed explanation of the up-regulation of genes, see RNAi:upregulation of genes
RNA silencing also gets regulated
The same way that RNA silencing regulates downstream target mRNAs, RNA silencing itself is regulated. For example, silencing signals get spread between cells by a group of enzymes called RdRPs (RNA-dependent RNA polymerases) or RDRs.[2]
Practical applications
Growing understanding of small RNA gene-silencing mechanisms involving dsRNA-mediated sequence-specific mRNA degradation has directly impacted the fields of functional genomics, biomedicine, and experimental biology. The following section describes various applications involving the effects of RNA silencing. These include uses in biotechnology, therapeutics, and laboratory research. Bioinformatics techniques are also being applied to identify and characterize large numbers of small RNAs and their targets.
Biotechnology
Artificial introduction of long dsRNAs or siRNAs has been adopted as a tool to inactivate gene expression, both in cultured cells and in living organisms.[2] Structural and functional resolution of small RNAs as the effectors of RNA silencing has had a direct impact on experimental biology. For example, dsRNA may be synthesized to have a specific sequence complementary to a gene of interest. Once introduced into a cell or biological system, it is recognized as exogenous genetic material and activates the corresponding RNA silencing pathway. This mechanism can be used to effect decreases in gene expression with respect to the target, useful for investigating loss of function for genes relative to a phenotype. That is, studying the phenotypic and/or physiologic effects of expression decreases can reveal the role of a gene product. The observable effects can be nuanced, such that some methods can distinguish between “knockdown” (decrease expression) and “knockout” (eliminate expression) of a gene.[47] RNA interference technologies have been noted recently as one of the most widely utilized techniques in functional genomics.[48] Screens developed using small RNAs have been used to identify genes involved in fundamental processes such as cell division, apoptosis and fat regulation.
Biomedicine
Since at least the mid-2000s, there has been intensifying interest in developing short interfering RNAs for biomedical and therapeutic applications.[49] Bolstering this interest is a growing number of experiments which have successfully demonstrated the clinical potential and safety of small RNAs for combatting diseases ranging from viral infections to cancer as well as neurodegenerative disorders.[50] In 2004, the first Investigational New Drug applications for siRNA were filed in the United States with the Food and Drug Administration; it was intended as a therapy for age-related macular degeneration.[48] RNA silencing in vitro and in vivo has been accomplished by creating triggers (nucleic acids that induce RNAi) either via expression in viruses or synthesis of oligonucleotides.[51] Optimistically many studies indicate that small RNA-based therapies may offer novel and potent weapons against pathogens and diseases where small molecule/pharmacologic and vaccine/biologic treatments have failed or proved less effective in the past.[49] However, it is also warned that the design and delivery of small RNA effector molecules should be carefully considered in order to ensure safety and efficacy.
The role of RNA silencing in therapeutics, clinical medicine, and diagnostics is a fast developing area and it is expected that in the next few years some of the compounds using this technology will reach market approval. A report has been summarized below to highlight the many clinical domains in which RNA silencing is playing an increasingly important role, chief among them are ocular and retinal disorders, cancer, kidney disorders, LDL lowering, and antiviral.[51] The following table displays a listing of RNAi based therapy currently in various phases of clinical trials. The status of these trials can be monitored on the ClinicalTrials.gov website, a service of the National Institutes of Health (NIH).[52] Of note are treatments in development for ocular and retinal disorders, that were among the first compounds to reach clinical development. AGN211745 (sirna027) (Allergan) and bevasiranib (Cand5) (Opko) underwent clinical development for the treatment of age-related macular degeneration, but trials were terminated before the compounds reached the market. Other compounds in development for ocular conditions include SYL040012 (Sylentis) and QPI-007 (Quark). SYL040012 (bamosinan) is a drug candidate under clinical development for glaucoma, a progressive optic neurdegeneration frequently associated to increased intraocular pressure; QPI-007 is a candidate for the treatment of angle-closure glaucoma and Non-arteritic anterior ischaemic optic neuropathy; both compounds are currently undergoing phase II clinical trials. Several compounds are also under development for conditions such as cancer and rare diseases.
| Clinical domain | Drug | Indication | Target |
|---|---|---|---|
| Ocular and retinal disorders | TD101 | Pachyonychia congenita | Keratin 6A N171K mutant |
| Ocular and retinal disorders | QPI-1007 | Non-arteritic anterior ischaemic optic neuropathy | Caspase 2 |
| Ocular and retinal disorders | AGN211745 | Age-related macular degeneration, choroidal neovascularization | VEGFR1 |
| Ocular and retinal disorders | PF-655 | Diabetic macular oedema, age-related macular degeneration | RTP801 |
| Ocular and retinal disorders | SYL040012 | Glaucoma | β2 adrenergic receptor |
| Ocular and retinal disorders | Bevasiranib | Diabetic macular oedema | VEGF |
| Ocular and retinal disorders | Bevasiranib | Macular degeneration | VEGF |
| Cancer | CEQ508 | Familial adenomatous polyposis | β-catenin |
| Cancer | ALN-PLK1 | Liver tumor | PLK1 |
| Cancer | FANG | Solid tumor | Furin |
| Cancer | CALAA-01 | Solid tumor | RRM2 |
| Cancer | SPC2996 | chronic lymphocytic leukemia | BCL-2 |
| Cancer | ALN-VSP02 | Solid tumor | VEGF, kinesin spindle protein |
| Cancer | NCT00672542 | Metastatic melanoma | LMP2, LMP7, and MECL1 |
| Cancer | Atu027 | Solid malignancies | PKN3 |
| Kidney disorders | QPI-1002/I5NP | Acute kidney injury | p53 |
| Kidney disorders | QPI-1002/I5NP | Graft dysfunction kidney transplant | p53 |
| Kidney disorders | QPI-1002/I5NP | Kidney injury acute renal failure | p53 |
| LDL lowering | TKM-ApoB | Hypercholesterolaemia | APOB |
| LDL lowering | PRO-040,201 | Hypercholesterolaemia | APOB |
| Antiviral | miravirsen | Hepatitis C virus | miR-122 |
| Antiviral | pHIV7-shI-TAR-CCR5RZ | HIV | HIV Tat protein, HIV TAR RNA, human CCR5 |
| Antiviral | ALN-RSV01 | RSV | RSV nucleocapsid |
| Antiviral | ALN-RSV01 | RSV in lung transplant patients | RSV nucleocapsid |
Main challenge
As with conventional manufactured drugs, the main challenge in developing successful offshoots of the RNAi-based drugs is the precise delivery of the RNAi triggers to where they are needed in the body. The reason that the ocular macular degeneration antidote was successful sooner than the antidote with other diseases is that the eyeball is almost a closed system, and the serum can be injected with a needle exactly where it needs to be. The future successful drugs will be the ones who are able to land where needed, probably with the help of nanobots. Below is a rendition of a table[51] that shows the existing means of delivery of the RNAi triggers.
| Species/formulation | Packaging capacity | Applications and considerations |
|---|---|---|
| Viral vector | ||
| Adenovirus | Usually < 10 Kb | dsDNA vector with large packaging capacity, transient expression, highly immunogenic |
| Adeno-associated virus (AAV) | ~4.5Kb | ssDNA vector, small packaging capacity, mildly immunogenic, lasting expression in non-dividing cells, capsid pseudotyping/engineering facilitates specific cell-targeting |
| Lentivirus | Up to 13.5 Kb | RNA vector, integration competent and incompetent forms available, less immunogenic than adenovirus or AAV, envelope pseudo typing facilitates cell targeting, clinical production more difficult than for adenovirus or AAV |
| Herpes simplexvirus | 150 Kb | DNA vector, episomal, lasting expression, immunogenic |
| Bacterial vector species (bacterial minicells can carry plasmids, siRNAs or drugs) | ||
| Escherichis coli, S. Typhymurium | Delivery of short hairpin RNA or siRNA to gut tissue | |
| Non-viral formulations | ||
| Nanoparticle | Self-assembling, may target specific receptors, requires technical expertise to prepare | |
| Stable nucleic acid lipid particle (SNALP) | Stable for systemic delivery, broad cell-tye delivery | |
| Aptamer | Targeting of specific receptors, requires sophisticated screening to develop | |
| Cholesterol | Stable for systemic delivery, broad cell-type delivery |
Laboratory
The scientific community has been quick to harness RNA silencing as a research tool. The strategic targeting of mRNA can provide a large amount of information about gene function and its ability to be turned on and off. Induced RNA silencing can serve as a controlled method for suppressing gene expression. Since the machinery is conserved across most eukaryotes, these experiments scale well to a range of model organisms.[53] In practice, expressing synthetic short hairpin RNAs can be used to reach stable knock-down.[54] If promoters can be made to express these designer short hairpin RNAs, the result is often potent, stable, and controlled gene knock-down in both in vitro and in vivo contexts.[55] Short hairpin RNA vector systems can be seen as roughly analogous in scope to using cDNA overexpression systems.[56] Overall, synthetic and natural small RNAs have proven to be an important tool for studying gene function in cells as well as animals.[57]
Bioinformatics approaches to identify small RNAs and their targets have returned several hundred, if not thousands of, small RNA candidates predicted to affect gene expression in plants, C. elegans, D. melanogaster, zebrafish, mouse, rat, and human.[58] These methods are largely directed to identifying small RNA candidates for knock-out experiments but may have broader applications. One bioinformatics approach evaluated sequence conservation criteria by filtering seed complementary target-binding sites. The cited study predicted that approximately one third of mammalian genes were to be regulated by, in this case, miRNAs.[59]
Ethics & Risk-Benefit Analysis
One aspect of RNA silencing to consider is its possible off-target affects, toxicity, and delivery methods. If RNA silencing is to become a conventional drug, it must first pass the typical ethical issues of biomedicine.[60] Using risk-benefit analysis, researchers can determine whether RNA silencing conforms to ethical ideologies such as nonmaleficence, beneficence, and autonomy.[61]
There is a risk of creating infection-competent viruses that could infect non-consenting people.[62] There is also a risk of affecting future generations based on these treatments. These two scenarios, in respect to autonomy, is possible unethical. At this moment, unsafe delivery methods and unintended aspects of vector viruses add to the argument against RNA silencing.[61]
In terms of off-target effects, siRNA can induce innate interferon responses, inhibit endogenous miRNAs through saturation, and may have complementary sequences to other non-target mRNAs. These off-targets could also have target up-regulations such as oncogenes and antiapoptotic genes. The toxicity of RNA silencing is still under review as there are conflicting reports.[61][62][63]
RNA silencing is quickly developing, because of that, the ethical issues need to be discussed further. With the knowledge of general ethical principles, we must continuously perform risk-benefit analysis.[61]
References
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (Feb 1998). "Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans". Nature. 391 (6669): 806–11. Bibcode:1998Natur.391..806F. doi:10.1038/35888. PMID 9486653.
- Meister G, Tuschl T (Sep 2004). "Mechanisms of gene silencing by double-stranded RNA" (PDF). Nature. 431 (7006): 343–9. Bibcode:2004Natur.431..343M. doi:10.1038/nature02873. PMID 15372041.
- Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC (Mar 2007). "A slicer-mediated mechanism for repeat-associated siRNA 5' end formation in Drosophila". Science. 315 (5818): 1587–90. Bibcode:2007Sci...315.1587G. doi:10.1126/science.1140494. PMID 17322028.
- Moazed D (Jan 2009). "Small RNAs in transcriptional gene silencing and genome defence". Nature. 457 (7228): 413–20. Bibcode:2009Natur.457..413M. doi:10.1038/nature07756. PMC 3246369. PMID 19158787.
- Pickford AS, Cogoni C (May 2003). "RNA-mediated gene silencing". Cellular and Molecular Life Sciences. 60 (5): 871–82. doi:10.1007/s00018-003-2245-2. PMID 12827277.
- Tijsterman M, Ketting RF, Plasterk RH (2002). "The genetics of RNA silencing". Annual Review of Genetics. 36: 489–519. doi:10.1146/annurev.genet.36.043002.091619. PMID 12429701.
- Malecová B, Morris KV (Apr 2010). "Transcriptional gene silencing through epigenetic changes mediated by non-coding RNAs". Current Opinion in Molecular Therapeutics. 12 (2): 214–22. PMC 2861437. PMID 20373265.
- Meister G, Landthaler M, Dorsett Y, Tuschl T (Mar 2004). "Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing". RNA. 10 (3): 544–50. doi:10.1261/rna.5235104. PMC 1370948. PMID 14970398.
- Zhou H, Hu H, Lai M (Dec 2010). "Non-coding RNAs and their epigenetic regulatory mechanisms". Biology of the Cell. 102 (12): 645–55. doi:10.1042/BC20100029. PMID 21077844.
- Ding SW (Apr 2000). "RNA silencing". Current Opinion in Biotechnology. 11 (2): 152–6. doi:10.1016/s0958-1669(00)00074-4. PMID 10753772.
- Susi P, Hohkuri M, Wahlroos T, Kilby NJ (Jan 2004). "Characteristics of RNA silencing in plants: similarities and differences across kingdoms". Plant Molecular Biology. 54 (2): 157–74. doi:10.1023/B:PLAN.0000028797.63168.a7. PMID 15159620.
- Baulcombe D (Sep 2004). "RNA silencing in plants". Nature. 431 (7006): 356–63. Bibcode:2004Natur.431..356B. doi:10.1038/nature02874. PMID 15372043.
- Baulcombe D (Jun 2005). "RNA silencing". Trends in Biochemical Sciences. 30 (6): 290–3. doi:10.1016/j.tibs.2005.04.012. PMID 15950871.
- Matzke MA, Birchler JA (Jan 2005). "RNAi-mediated pathways in the nucleus". Nature Reviews Genetics. 6 (1): 24–35. doi:10.1038/nrg1500. PMID 15630419.
- Voinnet O (Mar 2005). "Induction and suppression of RNA silencing: insights from viral infections". Nature Reviews Genetics. 6 (3): 206–20. doi:10.1038/nrg1555. PMID 15703763.
- Grewal SI, Rice JC (Jun 2004). "Regulation of heterochromatin by histone methylation and small RNAs". Current Opinion in Cell Biology. 16 (3): 230–8. doi:10.1016/j.ceb.2004.04.002. PMID 15145346.
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M (Nov 2004). "A pancreatic islet-specific microRNA regulates insulin secretion". Nature. 432 (7014): 226–30. Bibcode:2004Natur.432..226P. doi:10.1038/nature03076. PMID 15538371.
- Eccleston, Alex; Angela K. Eggleston (2004). "RNA Interference". Nature. 431 (7006): 338–42. Bibcode:2004Natur.431..337E. doi:10.1038/431337a. PMID 15372040.
- Eggleston AK (Jan 2009). "RNA silencing". Nature. 457 (7228): 395. Bibcode:2009Natur.457..395E. doi:10.1038/457395a. PMID 19158784.
- Takeshita F, Ochiya T (Aug 2006). "Therapeutic potential of RNA interference against cancer". Cancer Science. 97 (8): 689–96. doi:10.1111/j.1349-7006.2006.00234.x. PMID 16863503.
- Dykxhoorn DM, Novina CD, Sharp PA (Jun 2003). "Killing the messenger: short RNAs that silence gene expression". Nature Reviews Molecular Cell Biology. 4 (6): 457–67. doi:10.1038/nrm1129. PMID 12778125.
- Hammond SM, Caudy AA, Hannon GJ (Feb 2001). "Post-transcriptional gene silencing by double-stranded RNA". Nature Reviews Genetics. 2 (2): 110–9. doi:10.1038/35052556. PMID 11253050.
- Bühler M (Apr 2009). "RNA turnover and chromatin-dependent gene silencing". Chromosoma. 118 (2): 141–51. doi:10.1007/s00412-008-0195-z. PMID 19023586.
- Gonzalez S, Pisano DG, Serrano M (Aug 2008). "Mechanistic principles of chromatin remodeling guided by siRNAs and miRNAs". Cell Cycle. 7 (16): 2601–8. doi:10.4161/cc.7.16.6541. PMID 18719372.
- Kim JK, Gabel HW, Kamath RS, Tewari M, Pasquinelli A, Rual JF, Kennedy S, Dybbs M, Bertin N, Kaplan JM, Vidal M, Ruvkun G (May 2005). "Functional genomic analysis of RNA interference in C. elegans". Science. 308 (5725): 1164–7. Bibcode:2005Sci...308.1164K. doi:10.1126/science.1109267. PMID 15790806.
- Bühler M, Moazed D (Nov 2007). "Transcription and RNAi in heterochromatic gene silencing". Nature Structural & Molecular Biology. 14 (11): 1041–8. doi:10.1038/nsmb1315. PMID 17984966.
- Dombroski BA, Feng Q, Mathias SL, Sassaman DM, Scott AF, Kazazian HH, Boeke JD (Jul 1994). "An in vivo assay for the reverse transcriptase of human retrotransposon L1 in Saccharomyces cerevisiae". Molecular and Cellular Biology. 14 (7): 4485–92. doi:10.1128/mcb.14.7.4485. PMC 358820. PMID 7516468.
- Svoboda P (2008). RNA silencing in mammalian oocytes and early embryos. Current Topics in Microbiology and Immunology. 320. pp. 225–56. doi:10.1007/978-3-540-75157-1_11. ISBN 978-3-540-75156-4. PMID 18268847.
- Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, Xu J, Kittler EL, Zapp ML, Weng Z, Zamore PD (May 2008). "Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells". Science. 320 (5879): 1077–81. Bibcode:2008Sci...320.1077G. doi:10.1126/science.1157396. PMC 2953241. PMID 18403677.
- Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS (Jun 2005). "Post-transcriptional gene silencing by siRNAs and miRNAs". Current Opinion in Structural Biology. 15 (3): 331–41. doi:10.1016/j.sbi.2005.05.006. PMID 15925505.
- Bao N, Lye KW, Barton MK (Nov 2004). "MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methylation of the template chromosome". Developmental Cell. 7 (5): 653–62. doi:10.1016/j.devcel.2004.10.003. PMID 15525527.
- Zeng Y, Yi R, Cullen BR (Jan 2005). "Recognition and cleavage of primary microRNA precursors by the nuclear processing enzyme Drosha". The EMBO Journal. 24 (1): 138–48. doi:10.1038/sj.emboj.7600491. PMC 544904. PMID 15565168.
- Irvine DV, Zaratiegui M, Tolia NH, Goto DB, Chitwood DH, Vaughn MW, Joshua-Tor L, Martienssen RA (Aug 2006). "Argonaute slicing is required for heterochromatic silencing and spreading". Science. 313 (5790): 1134–7. Bibcode:2006Sci...313.1134I. doi:10.1126/science.1128813. PMID 16931764.
- miRBase.org
- Friedman RC, Farh KK, Burge CB, Bartel DP (Jan 2009). "Most mammalian mRNAs are conserved targets of microRNAs". Genome Research. 19 (1): 92–105. doi:10.1101/gr.082701.108. PMC 2612969. PMID 18955434.
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM (Feb 2005). "Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs". Nature. 433 (7027): 769–73. Bibcode:2005Natur.433..769L. doi:10.1038/nature03315. PMID 15685193.
- Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N (Sep 2008). "Widespread changes in protein synthesis induced by microRNAs". Nature. 455 (7209): 58–63. Bibcode:2008Natur.455...58S. doi:10.1038/nature07228. PMID 18668040.
- Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP (Sep 2008). "The impact of microRNAs on protein output". Nature. 455 (7209): 64–71. Bibcode:2008Natur.455...64B. doi:10.1038/nature07242. PMC 2745094. PMID 18668037.
- Palmero EI, de Campos SG, Campos M, de Souza NC, Guerreiro ID, Carvalho AL, Marques MM (Jul 2011). "Mechanisms and role of microRNA deregulation in cancer onset and progression". Genetics and Molecular Biology. 34 (3): 363–70. doi:10.1590/S1415-47572011000300001. PMC 3168173. PMID 21931505.
- Bernstein C, Bernstein H (May 2015). "Epigenetic reduction of DNA repair in progression to gastrointestinal cancer". World Journal of Gastrointestinal Oncology. 7 (5): 30–46. doi:10.4251/wjgo.v7.i5.30. PMC 4434036. PMID 25987950.
- Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L (2014). "Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders". Frontiers in Cellular Neuroscience. 8: 75. doi:10.3389/fncel.2014.00075. PMC 3949217. PMID 24653674.
- Mellios N, Sur M (2012). "The Emerging Role of microRNAs in Schizophrenia and Autism Spectrum Disorders". Frontiers in Psychiatry. 3: 39. doi:10.3389/fpsyt.2012.00039. PMC 3336189. PMID 22539927.
- Geaghan M, Cairns MJ (Aug 2015). "MicroRNA and Posttranscriptional Dysregulation in Psychiatry". Biological Psychiatry. 78 (4): 231–9. doi:10.1016/j.biopsych.2014.12.009. PMID 25636176.
- Klattenhoff C, Theurkauf W (Jan 2008). "Biogenesis and germline functions of piRNAs". Development. 135 (1): 3–9. doi:10.1242/dev.006486. PMID 18032451.
- Ishizu H, Siomi H, Siomi MC (Nov 2012). "Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines". Genes & Development. 26 (21): 2361–73. doi:10.1101/gad.203786.112. PMC 3489994. PMID 23124062.
- Matthew P Scott; Lodish, Harvey F.; Arnold Berk; Kaiser, Chris; Monty Krieger; Anthony Bretscher; Hidde Ploegh; Angelika Amon (2012). Molecular Cell Biology. San Francisco: W. H. Freeman. ISBN 978-1-4292-3413-9.
- Voorhoeve PM, Agami R (Jan 2003). "Knockdown stands up". Trends in Biotechnology. 21 (1): 2–4. doi:10.1016/S0167-7799(02)00002-1. PMID 12480342.
- Karagiannis TC, El-Osta A (Oct 2005). "RNA interference and potential therapeutic applications of short interfering RNAs". Cancer Gene Therapy. 12 (10): 787–95. doi:10.1038/sj.cgt.7700857. PMID 15891770.
- Kim DH, Rossi JJ (Mar 2007). "Strategies for silencing human disease using RNA interference". Nature Reviews Genetics. 8 (3): 173–84. doi:10.1038/nrg2006. PMID 17304245.
- Stevenson M (Oct 2004). "Therapeutic potential of RNA interference". The New England Journal of Medicine. 351 (17): 1772–7. doi:10.1056/NEJMra045004. PMID 15496626.
- Davidson BL, McCray PB (May 2011). "Current prospects for RNA interference-based therapies". Nature Reviews Genetics. 12 (5): 329–40. doi:10.1038/nrg2968. PMC 7097665. PMID 21499294.
- http://clinicaltrials.gov
- Zeng Y, Wagner EJ, Cullen BR (Jun 2002). "Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells". Molecular Cell. 9 (6): 1327–33. doi:10.1016/s1097-2765(02)00541-5. PMID 12086629.
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS (Apr 2002). "Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells". Genes & Development. 16 (8): 948–58. doi:10.1101/gad.981002. PMC 152352. PMID 11959843.
- Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ, Lowe SW (Nov 2005). "Probing tumor phenotypes using stable and regulated synthetic microRNA precursors". Nature Genetics. 37 (11): 1289–95. doi:10.1038/ng1651. PMID 16200064.
- Rigó G, Papdi C, Szabados L (2012). Transformation using controlled cDNA overexpression system. Methods in Molecular Biology. 913. pp. 277–90. doi:10.1007/978-1-61779-986-0_19. ISBN 978-1-61779-985-3. PMID 22895767.
- Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, Siolas D, Hu G, Paddison PJ, Schlabach MR, Sheth N, Bradshaw J, Burchard J, Kulkarni A, Cavet G, Sachidanandam R, McCombie WR, Cleary MA, Elledge SJ, Hannon GJ (Nov 2005). "Second-generation shRNA libraries covering the mouse and human genomes". Nature Genetics. 37 (11): 1281–8. doi:10.1038/ng1650. PMID 16200065.
- Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E (Jan 2005). "Phylogenetic shadowing and computational identification of human microRNA genes". Cell. 120 (1): 21–4. doi:10.1016/j.cell.2004.12.031. PMID 15652478.
- Lewis BP, Burge CB, Bartel DP (Jan 2005). "Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets". Cell. 120 (1): 15–20. doi:10.1016/j.cell.2004.12.035. PMID 15652477.
- Beauchamp, TL; Childress, JF (2009). Principles of Biomedical Ethics, 6th ed. Oxford: Oxford University Press.
- Ebbesen, Mette; Jensen, Thomas G.; Andersen, Svend; Pedersen, Finn Skou (2008-06-25). "Ethical Perspectives on RNA Interference Therapeutics". International Journal of Medical Sciences. 5 (3): 159–168. doi:10.7150/ijms.5.159. ISSN 1449-1907. PMC 2443345. PMID 18612370.
- Cullen, RC (2006). "Enhancing and Confirming the Specificity of RNAi Experiments". Nature Methods. 3 (9): 677–681. doi:10.1038/nmeth913. PMID 16929311.
- Elbashir, SM; Martinez, J; Patkaniowska, A; Lendeckel, 2; Tuschl, T (2001). "Functional Anatomy of siRNA for Mediating Efficient RNAi in Drosophilia melanogaster Embryo Lysate". EMBO Journal. 20 (23): 6877–88. doi:10.1093/emboj/20.23.6877. PMC 125328. PMID 11726523.CS1 maint: numeric names: authors list (link)
External links
- Nature Reviews animation explaining the mechanism of RNAi can be found here.