Queuine
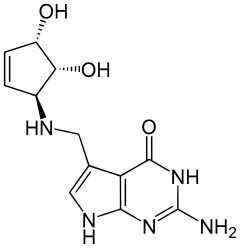
Queuine (/kjuːiːn/) (Q) is a hypermodified nucleobase found in the first (or wobble) position of the anticodon of tRNAs specific for Asn, Asp, His, and Tyr, in most eukaryotes and prokaryotes.[1]
 | |
| Names | |
|---|---|
| Other names
7-(((4,5-cis-dihydroxy-2-cyclopenten-1-yl)amino)methyl)-7-deazaguanine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | Queuine |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H15N5O3 | |
| Molar mass | 277.284 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The nucleoside of queuine is queuosine. Queuine is not found in the tRNA of archaea; however, a related 7-deazaguanine derivative, the nucleoside of which is archaeosine, occurs in different tRNA position, the dihydrouridine loop, and in tRNAs with more specificities.
History and Naming
In 1967, it was discovered that the four above-mentioned tRNAs contained an as-yet unknown nucleoside, which was designated "Nucleoside Q". This name remained in use throughout much of the work to characterize the compound, after which it was proposed that its common name should be based on the sound of the letter Q—thus producing "queuine" by analogy to guanine and other nucleobases, and "queuosine" by analogy to guanosine and other nucleosides.[2]
Biosynthesis and Function
Although queuosine is found in the tRNA of nearly all eukaryotic organisms, it is produced exclusively by bacteria; higher organisms must obtain queuine from the diet or salvage it from symbiotic microbes—a process for which dedicated enzymatic machinery exists.[3] As such, queuine has been described as a vitamin.[4] Because it can be generated from guanine by some species in the human microbiome, its status as a vitamin may be analogous to that of 4-Aminobenzoic acid, a "conditional" vitamin which is only essential in the diet if the microbiome does not produce sufficient quantities. As of 2019, human queuine requirements are not well understood, and the prevalence of queuine deficiency in humans is unknown.[5]
Once salvaged, queuine replaces a guanine base in the anticodon of certain tRNAs, where it appears to play a role in ensuring rapid and accurate recognition of the corresponding mRNAs' codons. In the absence of queuosine modification, translation at Q-decoded codons slows down to the point that many proteins cannot fold properly.[6] This biomolecular "traffic jam" triggers the unfolded protein response, a hallmark of various neurodegenerative diseases.
Role in Biopterin Recycling and Neurotransmitter Biosynthesis
The effects of queuine deficiency have been studied in germ-free animals, which have no intestinal microbiota. After removal of all queuine from the diet, germ-free mice lose the ability to convert the dietary amino acid phenylalanine into tyrosine–a phenotype mimicking the human disease phenylketonuria.[7]
Further investigation of this effect revealed it to be a consequence of impaired tetrahydrobiopterin (BH4) regeneration. BH4 is a cofactor for the biopterin-dependent aromatic amino acid hydroxylase enzymes, which catalyze the conversion of phenylalanine to tyrosine, tyrosine to L-DOPA, and tryptophan to 5-HTP, oxidizing BH4 to dihydrobiopterin (BH2) in the process. BH2 must then be converted back to BH4 by the enzyme dihydropteridine reductase before it can be used again. Queuine depletion appears to impair this "recycling" process, resulting in a deficit of BH4 and an excess of BH2, which in turn impairs the activity of the aromatic amino acid hydroxylase enzymes.[8]
Because these enzymes play a critical role in the biosynthesis of the neurotransmitters serotonin, melatonin, dopamine, norepinephrine, and epinephrine, problems with biopterin metabolism have long been considered a promising candidate to explain the origins of some psychiatric disorders characterized by imbalances in these neurotransmitters, including depression and schizophrenia. A number of studies suggest that these disorders are indeed associated with disrupted biopterin metabolism.[9][10] As queuine appears to be essential for the maintenance of adequate BH4 levels, queuine deficiency—resulting from disruption to the microbiome by factors like antibiotics—has been proposed as a possible cause of mental illnesses related to imbalances of these neurotransmitters.[5]
Role in Cancer
Although general queuine repletion status has not been studied in humans, studies of cancerous tissue have found a uniform trend of queuine deficiency in lung, ovarian, and lymphatic cancers.[11] In one study of patients with lung cancer, a lack of queuosine in tumor tissue tRNA was associated with worse odds of survival four years post-biopsy. [12] It has been postulated that this correlation is attributable to decreased activity of tRNA guanine transglycosylase, the enzyme which replaces guanine with queuine in tRNA.[13]
References
- Farkas, Walter R. (1983). "Queuine, the Q-Containing tRNAs and the Enzymes Responsible for Their Formation". Nucleosides and Nucleotides. 2: 1–20. doi:10.1080/07328318308078845.
- Nishimura, Susumu; et al. (1983). Cohn, Waldo (ed.). Progress in Nucleic Acid Research and Molecular Biology. 28. 111 Fifth Avenue, New York, New York 10003: Academic Press, Inc. pp. 50–80. ISBN 0-12-540028-4.CS1 maint: location (link)
- Zallot, Rémi; et al. (15 August 2014). "Plant, animal, and fungal micronutrient queuosine is salvaged by members of the DUF2419 protein family". ACS Chemical Biology. 9 (8): 1812–1825. doi:10.1021/cb500278k. PMC 4136680. PMID 24911101.
- Ames, Bruce (23 October 2018). "Prolonging healthy aging: Longevity vitamins and proteins". Proceedings of the National Academy of Sciences. 115 (43): 10836–10844. doi:10.1073/pnas.1809045115. PMC 6205492. PMID 30322941.
- Skolnick, Stephen; Greig, Nigel (1 March 2019). "Microbes and monoamines: Potential neuropsychiatric consequences of dysbiosis". Trends in Neurosciences. 42 (3): 151–163. doi:10.1016/j.tins.2018.12.005. PMID 30795845. Retrieved 6 May 2020.
- Tuorto, Francesca; et al. (14 September 2018). "Queuosine-modified tRNAs confer nutritional control of protein translation". EMBO J. 37 (18): e99777. doi:10.15252/embj.201899777. PMC 6138434. PMID 30093495.
- Marks, T.; Farkas, Walter (13 January 1997). "Effects of a diet deficient in tyrosine and queuine on germfree mice". Biochem. Biophys. Res. Commun. 230 (2): 233–237. doi:10.1006/bbrc.1996.5768. PMID 9016755.
- Rakovich, Tatsiana; et al. (12 April 2011). "Queuosine deficiency in eukaryotes compromises tyrosine production through increased tetrahydrobiopterin oxidation". Journal of Biological Chemistry. 286 (22): 19354–19363. doi:10.1074/jbc.M111.219576. PMID 9016755.
- Abou-Saleh, M.T.; et al. (1 October 1995). "The role of pterins in depression and the effects of antidepressive therapy". Biological Psychiatry. 38 (7): 458–463. doi:10.1016/0006-3223(94)00323-U. PMID 8672606. Retrieved 6 May 2020.
- Teraishi, T.; et al. (April 2018). "13C-phenylalanine breath test and serum biopterin in schizophrenia, bipolar disorder and major depressive disorder". Journal of Psychiatric Research. 99: 142–150. doi:10.1016/j.jpsychires.2018.01.019. PMID 29454221.
- Dirheimer, G.; et al. (1995). "Variations in tRNA modifications, particularly of their queuine content in higher eukaryotes. Its relation to malignancy grading". Biochimie. 77 (1–2): 99–103. doi:10.1016/0300-9084(96)88111-9. PMID 7599283. Retrieved 6 May 2020.
- Huang, Biing-Shiun; et al. (September 1992). "Relationship of the Queuine Content of Transfer Ribonucleic Acids to Histopathological Grading and Survival in Human Lung Cancer". Cancer Research. 52 (17): 99–103. doi:10.1016/0300-9084(96)88111-9. PMID 7599283. Retrieved 6 May 2020.
- Pathak, Chandramani; et al. (22 November 2005). "Hypomodification of Transfer RNA in Cancer with Respect to Queuosine". RNA Biology. 2 (4): 143–148. doi:10.4161/rna.2.4.2417. PMID 17114931. Retrieved 6 May 2020.
External links
- Human Metabolome Database: Queuine (HMDB01495)
- Akimoto, Hiroshi; Imamiya, Eiko; Hitaka, Takenori; Nomura, Hiroaki; Nishimura, Susumu (1988). "Synthesis of queuine, the base of naturally occurring hypermodified nucleoside (Queuosine), and its analogues". Journal of the Chemical Society, Perkin Transactions 1 (7): 1637. doi:10.1039/P19880001637.
- Barnett, Charles J.; Grubb, Lana M. (2000). "Total Synthesis of Q Base (Queuine)". Tetrahedron. 56 (47): 9221–9225. doi:10.1016/S0040-4020(00)00895-4.)
- Brooks, Allen F.; Garcia, George A.; Showalter, H.D. Hollis (2010). "A short, concise synthesis of queuine" (PDF). Tetrahedron Letters. 51 (32): 4163–4165. doi:10.1016/j.tetlet.2010.06.008.