Pyrenoid
Pyrenoids are sub-cellular micro-compartments found in chloroplasts of many algae,[1] and in a single group of land plants, the hornworts.[2] Pyrenoids are associated with the operation of a carbon-concentrating mechanism (CCM). Their main function is to act as centres of carbon dioxide (CO2) fixation, by generating and maintaining a CO2 rich environment around the photosynthetic enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO). Pyrenoids therefore seem to have a role analogous to that of carboxysomes in cyanobacteria.
Algae are restricted to aqueous environments, even in aquatic habitats, and this has implications for their ability to access CO2 for photosynthesis. CO2 diffuses 10,000 times slower in water than in air, and is also slow to equilibrate. The result of this is that water, as a medium, is often easily depleted of CO2 and is slow to gain CO2 from the air. Finally, CO2 equilibrates with bicarbonate (HCO3−) when dissolved in water, and does so on a pH-dependent basis. In sea water for example, the pH is such that dissolved inorganic carbon (DIC) is mainly found in the form of HCO3−. The net result of this is a low concentration of free CO2 that is barely sufficient for an algal RuBisCO to run at a quarter of its maximum velocity, and thus, CO2 availability may sometimes represent a major limitation of algal photosynthesis.
Discovery
Pyrenoids were first described in 1803 by Vaucher[3] (cited in Brown et al.[4]). The term was first coined by Schmitz [5] who also observed how algal chloroplasts formed de novo during cell division, leading Schimper to propose that chloroplasts were autonomous, and to surmise that all green plants had originated through the “unification of a colourless organism with one uniformly tinged with chlorophyll".[6] From these pioneering observations, Mereschkowski eventually proposed, in the early 20th century, the symbiogenetic theory and the genetic independence of chloroplasts.
In the following half-century, phycologists often used the pyrenoid as a taxonomic marker, but physiologists long failed to appreciate the importance of pyrenoids in aquatic photosynthesis. The classical paradigm, which prevailed until the early 1980s, was that the pyrenoid was the site of starch synthesis.[7] Microscopic observations were easily misleading as a starch sheath often encloses pyrenoids. The discovery of pyrenoid deficient mutants with normal starch grains in the green alga Chlamydomonas reinhardtii,[8] as well as starchless mutants with perfectly formed pyrenoids,[9] eventually discredited this hypothesis.
It was not before the early 1970s that the proteinaceous nature of the pyrenoid was elucidated, when pyrenoids were successfully isolated from a green alga,[10] and showed that up to 90% of it was composed of biochemically active RuBisCO. In the following decade, more and more evidence emerged that algae were capable of accumulating intracellular pools of DIC, and converting these to CO2, in concentrations far exceeding that of the surrounding medium. Badger and Price first suggested the function of the pyrenoid to be analogous to that of the carboxysome in cyanobacteria, in being associated with CCM activity.[11] CCM activity in algal and cyanobacterial photobionts of lichen associations was also identified using gas exchange and carbon isotope isotopes [12] and associated with the pyrenoid by Palmqvist [13] and Badger et al.[14] The Hornwort CCM was later characterized by Smith and Griffiths.[15]
From there on, the pyrenoid was studied in the wider context of carbon acquisition in algae, but has yet to be given a precise molecular definition.
Structure
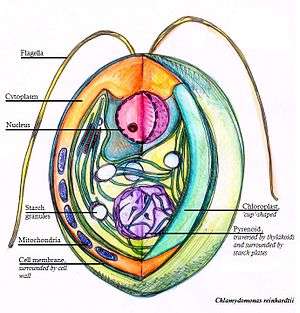
These contain protein beside starch.There is substantial diversity in pyrenoid morphology and ultrastructure between algal species. In the unicellular red alga Porphyridium purpureum and in the green alga Chlamydomonas reinhardtii, there is a single highly conspicuous pyrenoid in a single chloroplast, visible using light microscopy. By contrast, in diatoms and dinoflagellates, there can be multiple pyrenoids. When examined with transmission electron microscopy, pyrenoids appear as electron dense structures. The pyrenoid matrix, composed primarily of RuBisCO,[10] is often traversed by thylakoids, which are in continuity with stromal thylakoids. In Porphyridium, these transpyrenoidal thylakoids are naked;[16] in Chlamydomonas, they are seemingly encased in tubules.[17]
Unlike carboxysomes, pyrenoids are not delineated by a protein shell (or membrane). A starch sheath is often formed or deposited at the periphery of pyrenoids, even when that starch is synthesised in the cytosol rather than in the chloroplast.[18] In Chlamydomonas, a high-molecular weight complex of two proteins (LCIB/LCIC) forms an additional concentric layer around the pyrenoid, outside the starch sheath, and this is currently hypothesised to act as a barrier to CO2-leakage or to recapture CO2 that escapes from the pyrenoid.[19]
The entire protein diversity and composition of the pyrenoid has yet to be fully elucidated, but thus far, a number of proteins other than RuBisCO have been shown to localise to the pyrenoid; namely, rubisco activase,[20] nitrate reductase[21] and nitrite reductase.[22] However it is not yet known how the pyrenoid forms during cell division. Mutagenic work on Chlamydomonas has shown that the RuBisCO small subunit is important for pyrenoid assembly,[23] and that two solvent exposed alpha-helices of the RuBisCO small subunit are key to the process.[24] Whether RuBisCO self-assembles into pyrenoids or requires additional chaperones is at present not known.
Role of Pyrenoids in the CCM
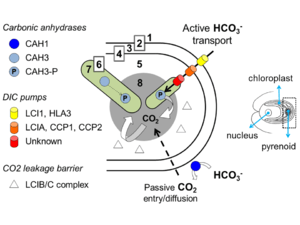
The confinement of the CO2-fixing enzyme into a subcellular micro-compartment, in association with a mechanism to deliver CO2 to that site, is believed to enhance the efficacy of photosynthesis in an aqueous environment. Having a CCM favours carboxylation over wasteful oxygenation by RuBisCO. The molecular basis of the pyrenoid and the CCM have been characterised to some detail in the model green alga Chlamydomonas reinhardtii.
The current model of the biophysical CCM reliant upon a pyrenoid[25][26] considers active transport of bicarbonate from the extracellular environment to the vicinity of RuBisCO, via transporters at the plasma membrane, the chloroplast membrane, and thylakoid membranes. Carbonic anhydrases in the periplasm and also in the cytoplasm and chloroplast stroma are thought to contribute to maintaining an intracellular pool of dissolved inorganic carbon, mainly in the form of bicarbonate. This bicarbonate is then thought to be pumped into the lumen of transpyrenoidal thylakoids, where a resident carbonic anhydrase is hypothesised to convert bicarbonate to CO2, and saturate RuBisCO with carboxylating substrate. It is likely that different algal groups evolved different types of CCMs, but it is generally taken that the algal CCM is articulated around a combination of carbonic anhydrases, inorganic carbon transporters, and some compartment to package RuBisCO.
Pyrenoids are highly plastic structures and the degree of RuBisCO packaging correlates with the state of induction of the CCM. In Chlamydomonas, when the CCM is repressed, for example when cells are maintained in a CO2-rich environment, the pyrenoid is small and the matrix is unstructured.[27] In the dinoflagellate Gonyaulax, the localisation of RuBisCO to the pyrenoid is under circadian control: when cells are photosynthetically active during the day, RuBisCO assembles into multiple chloroplasts at the centre of the cells; at night, these structures disappear.[28]
Physiology and regulation of the CCM
The algal CCM is inducible, and induction of the CCM is generally the result of low CO2 conditions. Induction and regulation of the Chlamydomonas CCM was recently studied by transcriptomic analysis, revealing that one out of three genes are up- or down-regulated in response to changed levels of CO2 in the environment.[29] Sensing of CO2 in Chlamydomonas involves a “master switch”, which was co-discovered by two laboratories.[30][31] This gene, Cia5/Ccm1, affects over 1,000 CO2-responsive genes [32] and also conditions the degree of packing of RuBisCO into the pyrenoid.
Origin
The CCM is only induced during periods of low CO2 levels, and it was the existence of these trigger levels of CO2 below which CCMs are induced that led researchers to speculate on the likely timing of origin of mechanisms like the pyrenoid.
There are several hypotheses as to the origin of pyrenoids. With the rise of large terrestrial based flora following the colonisation of land by ancestors of Charophyte algae, CO2 levels dropped dramatically, with a concomitant increase in O2 atmospheric concentration. It has been suggested that this sharp fall in CO2 levels acted as an evolutionary driver of CCM development, and thus gave rise to pyrenoids [33] in doing so ensuring that rate of supply of CO2 did not become a limiting factor for photosynthesis in the face of declining atmospheric CO2 levels.
However, alternative hypotheses have been proposed. Predictions of past CO2 levels suggest that they may have previously dropped as precipitously low as that seen during the expansion of land plants: approximately 300 MYA, during the Proterozoic Era.[34] This being the case, there might have been a similar evolutionary pressure that resulted in the development of the pyrenoid, though in this case, a pyrenoid or pyrenoid-like structure could have developed, and have been lost as CO2 levels then rose, only to be gained or developed again during the period of land colonisation by plants. Evidence of multiple gains and losses of pyrenoids over relatively short geological time spans was found in hornworts.[2]
Diversity
Pyrenoids are found in algal lineages,[1] irrespective of whether the chloroplast was inherited from a single endosymbiotic event (e.g. green and red algae, but not in glaucophytes) or multiple endosymbiotic events (diatoms, dinoflagellates, coccolithophores, cryptophytes, chlorarachniophytes, and euglenozoa. Some algal groups, however, lack pyrenoids altogether: "higher" red algae and extremophile red algae, the green alga genus Chloromonas, and "golden algae". Pyrenoids are usually considered to be poor taxonomic markers and may have evolved independently many times.[35]
References
- Giordano, M., Beardall, J., & Raven, J. A. (2005). CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol., 56, 99-131. PMID 15862091
- Villarreal, J. C., & Renner, S. S. (2012) Hornwort pyrenoids, carbon-concentrating structures, evolved and were lost at least five times during the last 100 million years. Proceedings of the National Academy of Sciences,109(46), 1873-1887. PMID 23115334
- Vaucher, J.-P. (1803). Histoire des conferves d'eau douce, contenant leurs différens modes de reproduction, et la description de leurs principales espèces, suivie de l'histoire des trémelles et des ulves d'eau douce. Geneva: J. J. Paschoud.
- Brown, R.M., Arnott, H.J., Bisalputra, T., and Hoffman, L.R. (1967). The pyrenoid: Its structure, distribution, and function. Journal of Phycology, 3(Suppl. 1), 5-7.
- Schmitz, F. (1882). Die Chromatophoren der Algen. Vergleichende untersuchungen über Bau und Entwicklung der Chlorophyllkörper und der analogen Farbstoffkörper der Algen. M. Cohen & Sohn (F. Cohen), Bonn, Germany.
- Schimper, A.F.W. (1883). Über die Entwicklung der Chlorophyllkörner und Farbkörper. Botanische Zeitung , 41, 105-120, 126-131, 137-160.
- Griffiths, D.J. (1980). The pyrenoid and its role in algal metabolism. Science Progress, 66, 537-553.
- Goodenough, U.W. and Levine, R.P. (1970). Chloroplast structure and function in AC-20, a mutant strain of Chlamydomonas reinhardtii. III. Chloroplast ribosomes and membrane organization. J Cell Biol , 44, 547-562.
- Villarejo, A., Plumed, M., and Ramazanov, Z. (1996). The induction of the CO2 concentrating mechanism in a starch-less mutant of Chlamydomonas reinhardtii. Physiol Plant, 98, 798-802.
- Holdsworth, R.H. (1971). The isolation and partial characterization of the pyrenoid protein of Eremosphaera viridis. J Cell Biol, 51, 499-513.
- Badger, M. R., & Price, G. D. (1992). The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiologia Plantarum, 84(4), 606-615.
- Máguas, C., Griffiths, H., Ehleringer, J., & Serodio, J. (1993). Characterization of photobiont associations in lichens using carbon isotope discrimination techniques. Stable Isotopes and Plant Carbon-Water Relations, 201-212.
- Palmqvist, K. (1993). Photosynthetic CO2-use efficiency in lichens and their isolated photobionts: the possible role of a CO2-concentrating mechanism. Planta, 191(1), 48-56.
- Badger, M. R., Pfanz, H., Büdel, B., Heber, U., & Lange, O. L. (1993). Evidence for the functioning of photosynthetic CO2-concentrating mechanisms in lichens containing green algal and cyanobacterial photobionts. Planta,191(1), 57-70.
- Smith, E. C., & Griffiths, H. (1996). A pyrenoid-based carbon-concentrating mechanism is present in terrestrial bryophytes of the class Anthocerotae. Planta, 200(2), 203-212.
- Brody, M., & Vatter, A. E. (1959). Observations on cellular structures of Porphyridium cruentum. The Journal of Biophysical and Biochemical Cytology,5(2), 289-294. PMID 13654450
- Sager, R., & Palade, G. E. (1957). Structure and development of the chloroplast in Chlamydomonas I. The normal green cell. The Journal of Biophysical and Biochemical Cytology, 3(3), 463-488.PMID 13438931
- Wilson, S., West, J., Pickett‐Heaps, J., Yokoyama, A., & Hara, Y. (2002). Chloroplast rotation and morphological plasticity of the unicellular alga Rhodosorus (Rhodophyta, Stylonematales). Phycological research, 50(3), 183-191.
- Yamano, T., Tsujikawa, T., Hatano, K., Ozawa, S. I., Takahashi, Y., & Fukuzawa, H. (2010). Light and low-CO2-dependent LCIB–LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant and Cell Physiology, 51(9), 1453-1468.PMID 20660228
- McKay, R. M. L., Gibbs, S. P., & Vaughn, K. C. (1991). RuBisCo activase is present in the pyrenoid of green algae. Protoplasma, 162(1), 38-45.
- Lopez-Ruiz, A., Verbelen, J. P., Roldan, J. M., & Diez, J. (1985). Nitrate reductase of green algae is located in the pyrenoid. Plant Physiology, 79(4), 1006-1010.
- López-Ruiz, A., Verbelen, J. P., Bocanegra, J. A., & Diez, J. (1991). Immunocytochemical localization of nitrite reductase in green algae. Plant Physiology, 96(3), 699-704.
- Genkov, T., Meyer, M., Griffiths, H., & Spreitzer, R. J. (2010). Functional hybrid rubisco enzymes with plant small subunits and algal large subunits engineered RBCS cDNA for expression in Chlamydomonas. Journal of Biological Chemistry,285(26), 19833-19841 PMID 20424165
- Meyer, M. T., Genkov, T., Skepper, J. N., Jouhet, J., Mitchell, M. C., Spreitzer, R. J., & Griffiths, H. (2012). RuBisCO small-subunit α-helices control pyrenoid formation in Chlamydomonas. Proceedings of the National Academy of Sciences, 109(47), 19474-19479. PMID 23112177
- Moroney, J. V., & Ynalvez, R. A. (2007). Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryotic cell, 6(8), 1251-1259. PMID 17557885
- Grossman, A. R., Croft, M., Gladyshev, V. N., Merchant, S. S., Posewitz, M. C., Prochnik, S., & Spalding, M. H. (2007). Novel metabolism in Chlamydomonas through the lens of genomics. Current Opinion in Plant Biology, 10(2), 190-198 PMID 17291820
- Rawat, M., Henk, M. C., Lavigne, L. L., & Moroney, J. V. (1996). Chlamydomonas reinhardtii mutants without ribulose-1, 5-bisphosphate carboxylase-oxygenase lack a detectable pyrenoid. Planta, 198(2), 263-270.
- Nassoury, N., Wang, Y., & Morse, D. (2005). Brefeldin a inhibits circadian remodeling of chloroplast structure in the dinoflagellate Gonyaulax. Traffic, 6(7), 548-561. PMID 15941407
- Brueggeman, A. J., Gangadharaiah, D. S., Cserhati, M. F., Casero, D., Weeks, D. P., & Ladunga, I. (2012). Activation of the carbon concentrating mechanism by CO2 deprivation coincides with massive transcriptional restructuring in Chlamydomonas reinhardtii. The Plant Cell, 24(5), 1860-1875 PMID 22634764
- Xiang, Y., Zhang, J., & Weeks, D. P. (2001). The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences, 98(9), 5341-5346 PMID 11309511
- Fukuzawa, H., Miura, K., Ishizaki, K., Kucho, K. I., Saito, T., Kohinata, T., & Ohyama, K. (2001). Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proceedings of the National Academy of Sciences, 98(9), 5347-5352. PMID 11287669
- Fang, W., Si, Y., Douglass, S., Casero, D., Merchant, S. S., Pellegrini, M., ... & Spalding, M. H. (2012). Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. The Plant Cell, 24(5), 1876-1893. PMID 22634760
- Badger, M. R., & Price, G. D. (2003). CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany, 54(383), 609-622. PMID 12554074
- Riding, R. (2006). Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic–Cambrian changes in atmospheric composition.Geobiology, 4(4), 299-316.
- Meyer, M., & Griffiths, H. (2013). Origins and diversity of eukaryotic CO2-concentrating mechanisms: lessons for the future. Journal of experimental botany, 64(3), 769-786 PMID 23345319.