Hydroxymethylbilane
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | hydroxymethylbilane |
PubChem CID |
|
| |
| |
| Properties | |
| C40H46N4O17 | |
| Molar mass | 854.81 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
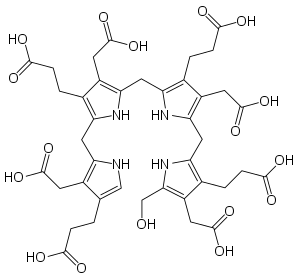
The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges −CH
2−. The chain starts with a hydroxymethyl group −CH
2−OH and ends with an hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group −CH
2−COOH and a propionic acid group −CH
2−CH
2−COOH, in that order. [1]
The compound is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase:
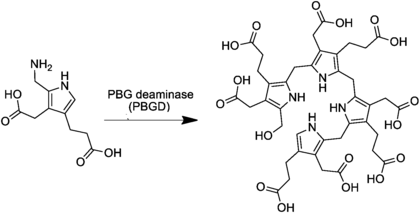
The enzyme uroporphyrinogen III synthase closes the chain to form a porphyrinogen a class of compounds with the hexahydroporphine macrocycle; specifically, uroporphyrinogen III. In the absence of the enzyme, the compound undergoes spontaneous cyclization and becomes uroporphyrinogen I.
References
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.