Precorrin-3B C17-methyltransferase
In enzymology, precorrin-3B C17-methyltransferase (EC 2.1.1.131) is an enzyme that catalyzes the chemical reaction
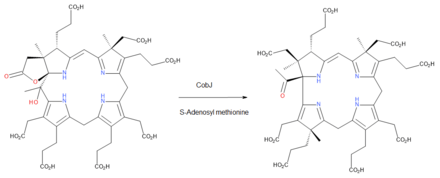
- S-adenosyl-L-methionine + precorrin-3B S-adenosyl-L-homocysteine + precorrin-4
| precorrin-3B C17-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.1.1.131 | ||||||||
| CAS number | 152787-64-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The two substrates of this enzyme are S-adenosyl methionine and precorrin 3B, and its two products are S-adenosylhomocysteine and precorrin 4.
This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:precorrin-3B C17-methyltransferase. Other names in common use include precorrin-3 methyltransferase, and CobJ. This enzyme is part of the biosynthetic pathway to cobalamin (vitamin B12) in aerobic bacteria and during this step the macrocycle ring-contracts so that the corrin core of the vitamin is formed.
See Also
References
- Scott AI, Roessner CA, Stolowich NJ, Spencer JB, Min C, Ozaki SI (1993). "Biosynthesis of vitamin B12. Discovery of the enzymes for oxidative ring contraction and insertion of the fourth methyl group". FEBS Lett. 331 (1–2): 105–8. doi:10.1016/0014-5793(93)80306-F. PMID 8405386.
- Debussche L, Thibaut D, Cameron B, Crouzet J, Blanche F (1993). "Biosynthesis of the corrin macrocycle of coenzyme B12 in Pseudomonas denitrificans". J. Bacteriol. 175 (22): 7430–40. PMC 206888. PMID 8226690.