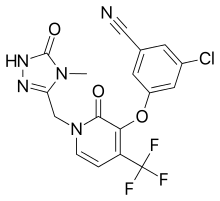
Doravirine
Doravirine (MK-1439) is a non-nucleoside reverse transcriptase inhibitor developed by Merck & Co. for use in the treatment of HIV/AIDS. In August 2018, the FDA approved doravirine under the product name Pifeltro, and in a combination tablet, doravirine/lamivudine/tenofovir disoproxil fumarate (Delstrigo).[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Pifeltro |
| Other names | MK-1439 |
| Routes of administration | Oral[1] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.234.454 |
| Chemical and physical data | |
| Formula | C17H11ClF3N5O3 |
| Molar mass | 425.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Collins, Simon; Horn, Tim. "The Antiretroviral Pipeline" (PDF). Pipeline Report. p. 10. Archived from the original (PDF) on 11 March 2016. Retrieved 6 December 2015.
- FDA Approves Merck’s DELSTRIGO™ (doravirine / lamivudine / tenofovir disoproxil fumarate), a Once-Daily Fixed-Dose Combination Tablet as a Complete Regimen and PIFELTRO™ (doravirine), an NNRTI, Both for the Treatment of HIV-1 in Appropriate Patients
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.