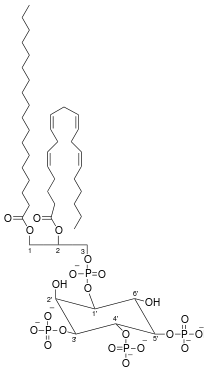
Phosphatidylinositol (3,4,5)-trisphosphate
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.
 | |
| Names | |
|---|---|
| Other names
PI(3,4,5)P3, PtdIns(3,4,5)P3 | |
| Identifiers | |
| Properties | |
| C47H86O22P4 | |
| Molar mass | 1126.46 g/mol, neutral with fatty acid composition - 18:0, 20:4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery
In 1988, Lewis C. Cantley published a paper describing the discovery of a novel type of phosphoinositide kinase with the unprecedented ability to phosphorylate the 3' position of the inositol ring resulting in the formation of phosphatidylinositol-3-phosphate (PI3P).[1] Working independently, Alexis Traynor-Kaplan and coworkers published a paper demonstrating that a novel lipid, phosphatidylinositol 3,4,5 trisphosphate (PIP3) occurs naturally in human neutrophils with levels that increased rapidly following physiologic stimulation with chemotactic peptide.[2] Subsequent studies demonstrated that in vivo the enzyme originally identified by Cantley's group prefers PtdIns(4,5)P2 as a substrate, producing the product PIP3.[3]
Function
PIP3 functions to activate downstream signaling components, the most notable one being the protein kinase AKT, which activates downstream anabolic signaling pathways required for cell growth and survival.
PtdIns(3,4,5)P3 is dephosphorylated by the phosphatase PTEN on the 3 position, generating PI(4,5)P2, and by SHIPs (SH2-containing inositol phosphatase) on the 5' position of the inositol ring, producing PI(3,4)P2.
The PH domain in a number of proteins binds to PtdIns(3,4,5)P3. Such proteins include Akt/PKB, PDK1, Btk1, and ARNO. The generation of PtdIns(3,4,5)P3 at the plasma membrane upon the activation of class I PI 3-kinases causes these proteins to translocate to the plasma membrane and affects their activity accordingly. In many types of eukaryotic cells, the production of PtdIns(3,4,5)P3 and recruitment of PH domain proteins to the membrane results in localized actin polymerization, leading to cellular protrusions that are important for cell migration, division, and phagocytosis.[4]
The PH domain allows binding between PtdIns(3,4,5)P3 and G protein-coupled receptor kinases (GRKs). This enhances the binding of the GRK to the plasma membrane.
References
- Whitman M, Downes CP, Keeler M, Keller T, Cantley L (April 1988). "Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate". Nature. 332 (6165): 644–6. doi:10.1038/332644a0. PMID 2833705.
- Traynor-Kaplan AE, Harris AL, Thompson BL, Taylor P, Sklar LA (July 1988). "An inositol tetrakisphosphate-containing phospholipid in activated neutrophils". Nature. 334 (6180): 353–6. doi:10.1038/334353a0. PMID 3393226.
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC (April 1989). "PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells". Cell. 57 (1): 167–75. doi:10.1016/0092-8674(89)90182-7. PMID 2467744.
- Artemenko Y, Lampert TJ, Devreotes PN (October 2014). "Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes". Cellular and Molecular Life Sciences. 71 (19): 3711–47. doi:10.1007/s00018-014-1638-8. PMC 4162842. PMID 24846395.