Bdelloidea
Bdelloidea /ˈdɛlɔɪdiə/ (Greek βδελλα, bdella, "leech-like") is a class of rotifers found in freshwater habitats all over the world. There are over 450 described species of bdelloid rotifers (or 'bdelloids'),[1] distinguished from each other mainly on the basis of morphology.[2] The main characteristics that distinguish bdelloids from related groups of rotifers are exclusively parthenogenetic reproduction and the ability to survive in dry, harsh environments by entering a state of desiccation-induced dormancy (anhydrobiosis) at any life stage.[3] They are often referred to as "ancient asexuals" due to their unique asexual history that spans back to over 25 million years ago through fossil evidence.[4] Bdelloid rotifers are microscopic organisms, typically between 150 and 700 µm in length.[3] Most are slightly too small to be seen with the naked eye, but appear as tiny white dots through even a weak hand lens, especially in bright light.
| Bdelloid rotifers | |
|---|---|
| SEM showing morphological variation of bdelloid rotifers and their jaws | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Rotifera |
| Superclass: | Eurotatoria |
| Class: | Bdelloidea Hudson, 1884 |
Evolutionary relationships
The phylum Rotifera traditionally included three classes: Bdelloidea, Monogononta and Seisonidea.[5] Prior to 1990, phylogenetic studies based on morphology seemed to indicate that the sister group to bdelloid rotifers was Monogononta, with seisonid rotifers as an early-diverging outgroup.[6]
.png)
Modern molecular phylogenetic studies demonstrate that this classic understanding of 'Rotifera' is incomplete (paraphyletic), because it omits a fourth clade of closely related organisms: the Acanthocephala, or thorny-headed worms.[9] Originally classified as a separate phylum, molecular and morphological evidence accumulated between 1994 and 2014 to indicate that Acanthocephala forms a monophyletic group with Bdelloidea, Monogononta and Seisonidea.[7][10] To accommodate this finding, some authors extend the term 'Rotifera' to include the highly modified, parasitic 'acanthocephalan rotifers' alongside bdelloid, monogonont and seisonid rotifers.[11] Others refer to the grouping of the four taxa as Syndermata, a term derived from their shared syncytial epidermis.[10]
The position of Bdelloidea within Syndermata (or Rotifera) is not entirely clear. Alternative possible phylogenetic relationships within the clade are illustrated by the accompanying cladograms. As of 2014, the "most comprehensive phylogenomic analysis of syndermatan relationships" to date was based on transcriptome data from all four groups,[7] and provided "strong support" for the hypothesis illustrated in the bottom left of the figure, in which Seisonidea and Acanthocephala are sister taxa. The study further indicated that the sister group to this taxon is Bdelloidea, whereas Monogononta is the outgroup to all three. This would mean that the closest living relatives of bdelloid rotifers are not monogonont rotifers, as previously believed, but seisonid rotifers and acanthocephalans, despite their highly modified morphology.
Classification and identification
Bdelloidea is a class of the phylum Rotifera, consisting of three orders: Philodinavida, Philodinida and Adinetida.[12] These orders are divided into four families and about 450 species.[13] Since these organisms are asexual the usual definition of a species as a group of organisms capable of creating fertile offspring is inapplicable, therefore the species concept in these organisms is based on a mixture of morphological and molecular data instead. DNA studies suggest that the diversity is much greater than the original morphological classifications suggest.[14][15]
Bdelloids can only be identified by eye while they are alive because many of the characteristics significant to classification are related to feeding and crawling; however, genetic identification of bdelloids is possible on dead individuals. Once preserved, the individuals contract into "blobs" which restricts analysis.[16] There are currently three morphological identification methodologies, two of which are considered dated: Bartoš (1951)[17] and Donner (1965).[13] The third method is a diagnostic key developed in 1995 by Shiel.[16]
Morphology
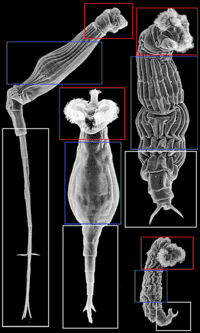
There are three main regions of the body of bdelloids: head, trunk and foot. The adjacent image depicts each area to show how body parts can be very different although they are named the same depending on the species involved. Bdelloids typically have a well-developed corona, divided into two parts, on a retractable head.
Some identifiable features of the bdelloids include :
- Well-developed foot glands[16]
- A mouth opening with a long oesophagus[16]
- Strong teeth (labelled by a tooth index)[16]
- Many cilia[16]
- Species-specific upper lip shape[16]
- Order-specific corona type[3]
- Philodinida consist of two ciliated discs
- Adinetida consist of a ventral ciliated field
- Philodinavida have a small corona
The bdelloid digestive and reproductive systems can be found within the trunk sections of their bodies, with the stomach being the most visible of the organs. In certain genera, (Habrotrocha, Otostephanos and Scepanotrocha) the bdelloid can actually be identified by the appearance of distinct spherical pellets within the stomach, which will be released as faeces. These pellets are a distinguishing characteristic since all the other genera release faeces as loose material.[3]
Most bdelloids retract the foot while they eat, but there are four genera that lack a foot: Adineta, Bradyscela, Henoceros and Philodinavus. This affects not only how they feed but also how they crawl; for instance Adineta and Bradyscela slide whereas the other genera loop.[3]
Behaviour
The behaviour of bdelloids can be split into four categories: feeding, locomotion, reproduction and stress-induced behaviours.
Feeding
The specific feeding behaviour of bdelloids is varied but most use rings of cilia in the corona organ to create currents of water which blow food through the mouth to the mastax organ which has been adapted specifically for grinding food.[18] Food includes suspended bacteria, algae, detritus, and other things.
Locomotion
There appear to be three main methods of movement: free swimming, inch-worming along a substrate, or sessility. Inch-worming (or crawling) involves taking alternate steps with the head and tail, as do certain leeches, which gives the group their name (Greek βδελλα or bdella, meaning leech). This video demonstrates how bdelloids move in three different situations: locomotion and feeding of bdelloid rotifers.
Reproduction
Bdelloids are of interest in the study of the evolution of sex because a male has never been observed,[19] and females reproduce exclusively by parthenogenesis, a form of asexual reproduction where embryos grow and develop without the need for fertilization; this is akin to the apomixis seen in some plants.[20] Each individual has paired gonads. Despite having been asexual for millions of years, they have diversified into more than 450 species and are fairly similar to other sexually reproducing rotifer species.
Evolution of obligate parthenogenetic reproduction
In 2003, the mode of asexual reproduction in the bdelloid rotifers was wholly unknown.[21] One theory of how obligate parthenogenesis arose in bdelloid rotifers was that parthenogenic lineages lost the ability to respond to sex-inducing signal, which is why these lineages retained their asexuality.[22] The obligate parthenogenetic strains of bdelloid rotifers produce a sex-inducing signal but have lost the ability to respond to that signal. It was later discovered that the inability to respond to sex-inducing signals in obligate parthenogens was caused by simple Mendelian inheritance of the gene op. [23]
Stress-induced behaviour
Bdelloids are able to survive environmental stresses by entering a state of dormancy known as anhydrobiosis which enables the organism to rapidly dehydrate and thus resist desiccation. While preparing for this dormant state many metabolic processes are adjusted to equate for the change in state; e.g. the production of protective chemicals.[24] The bdelloid can remain in this state, which is known as a 'xerosome' until the return of a sufficient amount of water, at which point they will rehydrate and become active within hours. Hatching of the young will only occur when conditions are at their most favourable.[25] These forms of dormancy are also known as cryptobiosis or quiescence. Bdelloids have been known to survive in this state for up to 9 years while waiting for favourable conditions to return.[25] In addition to surviving desiccation through anhydrobiosis, desiccation stress on two bdelloid species actually helped to maintain fitness and even improved their species fecundity.[26] The rotifers that were consistently kept hydrated fared worse than those desiccated and rehydrated.[27]
Bdelloidea have evolved a unique mechanism to help overcome one of the major perils of asexual reproduction. According to the Red Queen hypothesis of co-evolution, obligate asexuals will be driven extinct by rapidly changing parasites and pathogens, because they cannot change their genotypes quickly enough to keep up in this never-ending race. In populations of bdelloid rotifers, however, many parasites are destroyed during periods of extended desiccation.[28] Moreover, desiccated bdelloid rotifers are easily blown away from parasite-infested habitats by wind, and establish new, healthy populations elsewhere, which allows them to escape the Red Queen by moving in time and space instead of using sex to change their genotype.[29]
When these creatures recover from desiccation, it has been shown that they undergo a potentially unique genetic process where horizontal gene transfer occurs, resulting in a significant proportion of the bdelloid genome, up to 10%, having been obtained through horizontal gene transfer from bacteria, fungi and plants.[30] How and why horizontal gene transfer occur in bdelloids is under much debate at present; particularly with regards to possible connections between the foreign genes and the desiccation process as well as possible connections to bdelloids' ancient asexuality.
Bdelloid rotifers are extraordinarily resistant to damage from ionizing radiation due to the same DNA-preserving adaptations used to survive dormancy.[31] These adaptations include an extremely efficient mechanism for repairing DNA double-strand breaks.[32] This repair mechanism was studied in two Bdelloidea species, Adineta vaga,[32] and Philodina roseola.[33] and appears to involve mitotic recombination between homologous DNA regions within each species.
Horizontal gene transfer
Large-scale horizontal transfer of bacterial, plant and fungal genes into bdelloid rotifers[34] has been documented, and may represent an important factor in bdelloid evolution.
Gallery
 Lateral view of a bdelloid.
Lateral view of a bdelloid. Frontal view of a bdelloid's corona.
Frontal view of a bdelloid's corona..jpg) Lateral view of a bdelloid.
Lateral view of a bdelloid. Lateral view of a bdelloid.
Lateral view of a bdelloid.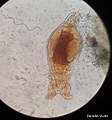 Lateral view of a bdelloid in algae-rich water
Lateral view of a bdelloid in algae-rich water
References
- Donner, Josef (1965). "Ordnung Bdelloidea (Rotatoria, Rädertiere)". Bestimmungsbücher zur Bodenfauna Europas, volume 6. Berlin: Akademie Verlag. OCLC 6733231. and Segers, Hendrik (2007). "Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution". Zootaxa. 1590: 3–104. Abstract.
- Segers, Hendrick (2007). Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution (PDF). Auckland: Magnolia Press. ISBN 978-1-86977-129-4.
- Ricci, Claudia (2000). "Key to the identification of the genera of bdelloid rotifers". Hydrobiologia. 418: 73–80. doi:10.1023/A:1003840216827.
- Poinar Jr., G. O.; Ricci, Claudia (1992). "Bdelloid rotifers in Dominican amber: Evidence for parthenogenetic continuity". Experientia. 48 (4): 408–410. doi:10.1007/BF01923444.
- King, Charles E.; Ricci, Claudia; Schonfeld, Justin; Serra, Manuel (September 2005). "Evolutionary Dynamics of 'the' Bdelloid and Monogonont Rotifer Life-history Patterns". Hydrobiologia. 546 (1): 55–70. CiteSeerX 10.1.1.455.6499. doi:10.1007/s10750-005-4102-9.
- Wallace, Robert Lee; Colburn, Rebecca Arlene (December 1989). "Phylogenetic relationships within phylum Rotifera: orders and genus Notholca". Hydrobiologia. 186–187 (1): 311–318. doi:10.1007/BF00048926.
- Wey-Fabrizius, Alexandra R.; Herlyn, Holger; Rieger, Benjamin; Rosenkranz, David; Witek, Alexander; Welch, David B. Mark; Ebersberger, Ingo; Hankeln, Thomas; Schmitz, Jürgen (10 February 2014). "Transcriptome Data Reveal Syndermatan Relationships and Suggest the Evolution of Endoparasitism in Acanthocephala via an Epizoic Stage". PLoS ONE. 9 (2): e88618. Bibcode:2014PLoSO...988618W. doi:10.1371/journal.pone.0088618. PMC 3919803. PMID 24520404.
- Lasek-Nesselquist, Erica (23 August 2012). "A Mitogenomic Re-Evaluation of the Bdelloid Phylogeny and Relationships among the Syndermata". PLoS ONE. 7 (8): e43554. Bibcode:2012PLoSO...743554L. doi:10.1371/journal.pone.0043554. PMC 3426538. PMID 22927990.
- Welch, David B. Mark (2001). "Early contributions of molecular phylogenetics to understanding the evolution of Rotifera". Hydrobiologia. 446: 315–322. doi:10.1023/A:1017502923286.
- Ahlrichs, Wilko H. (1997). "Epidermal ultrastructure of Seison nebaliae and Seison annulatus, and a comparison of epidermal structures within the Gnathifera". Zoomorphology. 117 (1): 41–48. doi:10.1007/s004350050028.
- Nielsen, Claus (2012). Animal evolution : interrelationships of the living phyla (3rd ed.). Oxford: Oxford University Press. ISBN 978-0199606030.
- Melone, Giulio & Ricci, Claudia (1995). "Rotatory apparatus in Bdelloids". Hydrobiologia. 313 (1): 91–98. doi:10.1007/BF00025935.
- Donner, Josef (1965). Ordnung Bdelloidea. Akademie-Verlag. p. 297. ISBN 9789031908851.
- Kaya, Murat; Herniou, Elisabeth A.; Barraclough, Timothy G. & Fontaneto, Diego (2009). "Inconsistent estimates of diversity between traditional and DNA taxonomy in bdelloid rotifers". Organisms Diversity & Evolution. 9 (1): 3–12. doi:10.1016/j.ode.2008.10.002.
- Fontaneto, Diego; Kaya, Murat; Herniou, Elisabeth A. & Barraclough, Timothy G. (2009). "Extreme levels of hidden diversity in microscopic animals (Rotifera) revealed by DNA taxonomy". Molecular Phylogenetics and Evolution. 53 (1): 182–189. doi:10.1016/j.ympev.2009.04.011. PMID 19398026.
- Shiel, Russell J. (1995). A guide to identification of rotifers, cladocerans and copepods from Australian inland waters. Australia: Co-operative Research Centre for Freshwater Ecology. ISBN 978-0-646-22410-7.
- Bartoš, Emanuel (1951). "The Czechoslovak Rotatoria of the order Bdelloidea". Memoires de la Societe Zoologique Tchecoslovaque de Prague. 15: 241–500.
- Klusemann, J.; Kleinow, W.; Peters, W. (1990). "The hard parts (trophi) of the rotifer mastax do contain chitin: evidence from studies on Brachionus plicatilis". Histochem. Cell Biol. 94 (3): 277–283. doi:10.1007/bf00266628. PMID 2401635.
- Judson, Olivia P.; Normark, Benjamin B. (1996). "Ancient asexual scandals". Trends in Ecology & Evolution. 11 (2): 41–46. doi:10.1016/0169-5347(96)81040-8. PMID 21237759.
- Milius, Susan (1 November 2002). "Bdelloids: No sex for over 40 million years". Science News. Retrieved 6 November 2016.
- Simon, Jean-Christophe; Delmotte, François; Rispe, Claude; Crease, Teresa (2003). "Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals". Biological Journal of the Linnean Society. 79 (1): 151–163. doi:10.1046/j.1095-8312.2003.00175.x.
- Stelzer, Claus-Peter (2008). "Obligate asex in a rotifer and the role of sexual signals". Journal of Evolutionary Biology. 21 (1): 287–293. doi:10.1111/j.1420-9101.2007.01437.x. PMID 17995949.
- Stelzer, Claus-Peter; Schmidt, Johanna; Wiedlroither, Anneliese; Riss, Simone (2010-09-20). "Loss of Sexual Reproduction and Dwarfing in a Small Metazoan". PLoS ONE. 5 (9): e12854. Bibcode:2010PLoSO...512854S. doi:10.1371/journal.pone.0012854. PMC 2942836. PMID 20862222.
- Crowe, John H. (1971). "Anhydrobiosis: an unsolved problem". American Naturalist. 105 (946): 563–573. doi:10.1086/282745. JSTOR 2459752.
- Guidetti, Roberto & Jönsson, K. Ingemar (2002). "Long-term anhydrobiotic survival in semi-terrestrial micrometazoans". Journal of Zoology. 257 (2): 181–187. CiteSeerX 10.1.1.630.9839. doi:10.1017/s095283690200078x.
- Ricci, Claudia & Fontaneto, Diego (2009). "The importance of being a bdelloid: ecological and evolutionary consequences of dormancy". Italian Journal of Zoology. 76 (3): 240–249. doi:10.1080/11250000902773484.
- Ricci, Claudia; Caprioli, Manuela; Fontaneto, Diego (2007). "Stress and fitness in parthenogens: is dormancy a key feature for bdelloid rotifers?". BMC Evolutionary Biology. 7 (Suppl 2): S9. doi:10.1186/1471-2148-7-S2-S9. PMC 1963474. PMID 17767737.
- Wilson, Christopher G.; Sherman, Paul W. (29 January 2010). "Anciently Asexual Bdelloid Rotifers Escape Lethal Fungal Parasites by Drying Up and Blowing Away". Science. 327 (5965): 574–576. Bibcode:2010Sci...327..574W. doi:10.1126/science.1179252. PMID 20110504.
- Wilson, Christopher G.; Sherman, Paul W. (22 August 2013). "Spatial and temporal escape from fungal parasitism in natural communities of anciently asexual bdelloid rotifers". Proceedings of the Royal Society B. 280 (1765): 20131255. doi:10.1098/rspb.2013.1255. PMC 3712457. PMID 23825214.
- Boschetti, Chiara; Pouchkina-Stantcheva, Natalia; Hoffmann, Pia & Tunnacliffe, Alan (2011). "Foreign genes and novel hydrophilic protein genes participate in the desiccation response of the bdelloid rotifer Adineta ricciae". The Journal of Experimental Biology. 214 (1): 59–68. doi:10.1242/jeb.050328. PMID 21147969.
- Gladyshev, Eugene & Meselson, Matthew (1 April 2008). "Extreme resistance of bdelloid rotifers to ionizing radiation". Proceedings of the National Academy of Sciences. 105 (13): 5139–5144. Bibcode:2008PNAS..105.5139G. doi:10.1073/pnas.0800966105. PMC 2278216. PMID 18362355.
- Hespeels B, Knapen M, Hanot-Mambres D, Heuskin AC, Pineux F, LUCAS S, Koszul R, Van Doninck K (July 2014). "Gateway to genetic exchange? DNA double-strand breaks in the bdelloid rotifer Adineta vaga submitted to desiccation". J. Evol. Biol. 27 (7): 1334–45. doi:10.1111/jeb.12326. PMID 25105197.
- Welch, David B. Mark; Welch, Jessica L. Mark & Meselson, Matthew (1 April 2008). "Evidence for degenerate tetraploidy in bdelloid rotifers". Proceedings of the National Academy of Sciences. 105 (13): 5145–9. Bibcode:2008PNAS..105.5145M. doi:10.1073/pnas.0800972105. PMC 2278229. PMID 18362354.
- Gladyshev, Eugene A.; Meselson, Matthew; Arkhipova, Irina R. (2008-05-30). "Massive horizontal gene transfer in bdelloid rotifers". Science. 320 (5880): 1210–1213. Bibcode:2008Sci...320.1210G. doi:10.1126/science.1156407. ISSN 1095-9203. PMID 18511688.
External links
| Wikimedia Commons has media related to Bdelloidea. |