Pentetic acid
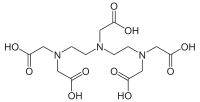
Pentetic acid or diethylenetriaminepentaacetic acid (DTPA) is an aminopolycarboxylic acid consisting of a diethylenetriamine backbone with five carboxymethyl groups. The molecule can be viewed as an expanded version of EDTA and is used similarly. It is a white solid with limited solubility in water.
 | |
| Names | |
|---|---|
| IUPAC name
2-[Bis[2-[bis(carboxymethyl)amino]ethyl]amino]acetic acid | |
| Other names
DTPA; H5dtpa; Diethylenetriaminepentaacetic acid; Penta(carboxymethyl)diethylenetriamine[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.593 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H23N3O10 | |
| Molar mass | 393.349 g·mol−1 |
| Appearance | White crystalline solid |
| Melting point | 220 °C (428 °F; 493 K) |
| Boiling point | decomposes at a higher temp. |
| <0.5 g/100 mL | |
| Acidity (pKa) | ~1.80 (20 °C) [2] |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Related compounds |
EDTA, NTA |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coordination properties
The conjugate base of DTPA has a high affinity for metal cations. Thus, the penta-anion DTPA5− is potentially an octadentate ligand assuming that each nitrogen centre and each COO−-group counts as a centre for coordination. The formation constants for its complexes are about 100 greater than those for EDTA.[3] As a chelating agent, DTPA wraps around a metal ion by forming up to eight bonds. Its complexes can also have an extra water molecule that coordinates the metal ion.[4] Transition metals, however, usually form less than eight coordination bonds. So, after forming a complex with a metal, DTPA still has the ability to bind to other reagents, as is shown by its derivative pendetide. For example, in its complex with copper(II), DTPA binds in a hexadentate manner utilizing the three amine centres and three of the five carboxylates.[5]
Applications
Like the more common EDTA, DTPA is predominantly used for sequestering metal ions that otherwise decompose hydrogen peroxide, which is used to bleach pulp in paper making. Several million kilograms are produced for this purpose annually.[3]
Its chelating properties are useful in deactivating calcium and magnesium ions in hair products. DTPA is used in over 150 cosmetic products.[6] Additionally, DTPA is used in MRI contrasting agents. DTPA improves MRI images by forming a complex with a gadolinium ion, which alters the properties of nearby water molecules.[7]
DTPA has been considered for treatment of radioactive materials such as plutonium, americium, and other actinides.[8] In theory, these complexes are more apt to be eliminated in urine. It is normally administered as the calcium or zinc salt, since these ions are readily displaced by more highly charged cations. DTPA forms complexes with thorium(IV), uranium(IV), neptunium(IV), and cerium(III/IV).[9]
In August, 2004 the US Food and Drug Administration (USFDA) determined zinc-DTPA and calcium-DTPA to be safe and effective for treatment of those who have breathed in or otherwise been contaminated internally by plutonium, americium, or curium. The recommended treatment is for an initial dose of calcium-DTPA, as this salt of DTPA has been shown to be more effective in the first 24 hours after internal contamination by plutonium, americium, or curium. After that time has elapsed both calcium-DTPA and zinc-DTPA are similarly effective in reducing internal contamination with plutonium, americium or curium, and zinc-DTPA is less likely to deplete the body's normal levels of zinc and other metals essential to health. Each drug can be administered by nebulizer for those who have breathed in contamination, and by intravenous injection for those contaminated by other routes.[10]
DTPA is also used as a chelate for aquarium plant fertilizer, specifically iron, an essential micronutrient typically needed in substantial quantities by all plants. Chelates are dissolved organic substances that bind to metals and prevent them from forming larger molecules through oxidation. FeDTPA is often sold under the name iron chelate 10% or 11% when used for the purpose of aquarium plant fertilization. Iron typically found in the aquarium water column has been converted into the ferric state (Fe3+) since it is in the presence of dissolved oxygen. However plants require iron in the ferrous state (Fe2+), therefore additional energy must be expended in order to extract the ferric iron from the water column and convert it to the ferrous form. When used to chelate iron fertilizer DTPA ensures that the iron is kept in the ferrous state (Fe2+) over time so it can be utilized by aquatic plants without expending valuable energy.
Related compounds
Compounds that are structurally related to DTPA are used in medicine, taking advantage of the high affinity of the triaminopentacarboxylate scaffold for metal ions.
- In ibritumomab tiuxetan, the chelator tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.[11]
- In capromab pendetide and satumomab pendetide, the chelator pendetide (GYK-DTPA) is a modified DTPA containing a peptide linker used to connect the chelate to an antibody.[12]
- Pentetreotide is a modified DTPA attached to a peptide segment.[13]
- DTPA and derivatives are used to chelate gadolinium to form a MRI contrast agent, such as Magnevist.
- Technetium-99m is chelated with DTPA for ventilation perfusion (V/Q) scans and radioisotope renography nuclear medicine scans.[14]
See also
References
- Anonymous Pentetic Acid. In Dictionary of Organic Compounds, Sixth Edition; Buckingham, J., Macdonald, F., Eds.; CRC Press: 1996; Vol. 5, pp 1188.
- Moeller, T.; Thompson, L. C. Observations on the rare earths—LXXV(1): The stabilities of diethylenetriaminepentaacetic acid chelates. Journal of Inorganic and Nuclear Chemistry 1962, 24, 499.
- J. Roger Hart "Ethylenediaminetetraacetic Acid and Related Chelating Agents" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a10_095
- Deblonde, Gauthier J.-P.; Kelley, Morgan P.; Su, Jing; Batista, Enrique R.; Yang, Ping; Booth, Corwin H.; Abergel, Rebecca J. (2018). "Spectroscopic and Computational Characterization of Diethylenetriaminepentaacetic Acid/Transplutonium Chelates: Evidencing Heterogeneity in the Heavy Actinide(III) Series". Angewandte Chemie International Edition. 57 (17): 4521–4526. doi:10.1002/anie.201709183. ISSN 1521-3773. PMID 29473263.
- V. V. Fomenko, T. N. Polynova, M. A. Porai-Koshits, G. L. Varlamova and N. I. Pechurova Crystal structure of copper (II) diethylenetriaminepentaacetate monohydrate Journal of Structural Chemistry, 1973, Vol. 14, 529. doi:10.1007/BF00747020
- Burnett, L. C. "Final Report on the Safety Assessment of Pentasodium Pentetate and Pentetic Acid as Used in Cosmetics" International Journal of Toxicology 2008, 27, 71-92.
- Caravan, Peter; Ellison, Jeffrey J.; McMurry, Thomas J. ; Lauffer, Randall B. "Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications" Chem. Revs. 1999, volume 99, pp. 2293–2342.
- Deblonde, Gauthier J.-P.; Kelley, Morgan P.; Su, Jing; Batista, Enrique R.; Yang, Ping; Booth, Corwin H.; Abergel, Rebecca J. (2018). "Spectroscopic and Computational Characterization of Diethylenetriaminepentaacetic Acid/Transplutonium Chelates: Evidencing Heterogeneity in the Heavy Actinide(III) Series". Angewandte Chemie International Edition. 57 (17): 4521–4526. doi:10.1002/anie.201709183. ISSN 1521-3773. PMID 29473263.
- (2) Brown, M. A.; Paulenova, A.; Gelis, A. V. "Aqueous Complexation of Thorium(IV), Uranium(IV), Neptunium(IV), Plutonium(III/IV), and Cerium(III/IV) with DTPA" Inorganic Chemistry 2012, volume 51, 7741-7748. doi:10.1021/ic300757k
- ""FDA Approves Drugs to Treat Internal Contamination from Radioactive Elements" (press release)". United States Food and Drug Administration. 19 June 2015 [4 August 2004]. Retrieved 2 August 2016.
- Milenic, Diane E.; Erik D. Brady; Martin W. Brechbiel (June 2004). "Antibody-targeted radiation cancer therapy". Nat Rev Drug Discov. 3 (6): 488–99. doi:10.1038/nrd1413. ISSN 1474-1776. PMID 15173838.
- Kahn, Daniel; J. Christopher Austin; Robert T Maguire; Sara J Miller; Jack Gerstbrein; Richard D Williams (1999). "A Phase II Study of [90Y] Yttrium-Capromab Pendetide in the Treatment of Men with Prostate Cancer Recurrence Following Radical Prostatectomy". Cancer Biotherapy & Radiopharmaceuticals. 14 (2): 99–111. doi:10.1089/cbr.1999.14.99. PMID 10850293.
- Liu, Shuang (2008-09-15). "Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides". Advanced Drug Delivery Reviews. 60 (12): 1347–70. doi:10.1016/j.addr.2008.04.006. ISSN 0169-409X. PMC 2539110. PMID 18538888.
- Chowdhury, Rajat; Wilson, Iain; Rofe, Christopher; Lloyd-Jones, Graham (2013-07-08). Radiology at a Glance. John Wiley & Sons. p. 109. ISBN 9781118691083.
- This article incorporates material from Facts about DTPA, a fact sheet produced by the United States Centers for Disease Control and Prevention.