Oxygen cycle
The oxygen cycle is the biogeochemical transitions of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth.[1] The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle.[2] Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).[1][2]
Reservoirs
Oxygen is one of the most abundant elements on Earth and represents a large portion of each main reservoir. By far the largest reservoir of Earth's oxygen is within the silicate and oxide minerals of the crust and mantle (99.5% by weight).[6] The Earth's atmosphere, hydrosphere, and biosphere together hold less than 0.05% of the Earth's total mass of oxygen. Besides O2, additional oxygen atoms are present in various forms spread throughout the surface reservoirs in the molecules of biomass, H2O, CO2, HNO3, NO, NO2, CO, H2O2, O3, SO2, H2SO4, MgO, CaO, AlO, SiO2, and PO4.[7]
Atmosphere
The atmosphere is 20.9% oxygen by volume, which equates to a total of roughly 34 × 1018 mol of oxygen.[2] Other oxygen-containing molecules in the atmosphere include ozone (O3), carbon dioxide (CO2), water vapor (H2O), and sulphur and nitrogen oxides (SO2, NO, N2O, etc.).
Biosphere
The biosphere is 22% oxygen by volume present mainly as a component of organic molecules (CxHxNxOx) and water molecules.
Hydrosphere
The hydrosphere is 33% oxygen by volume present mainly as a component of water molecules with dissolved molecules including free oxygen and carbonic acids (HxCO3).
Lithosphere
The lithosphere is 46.6% oxygen by volume present mainly as silica minerals (SiO2) and other oxide minerals.
Sources and sinks
While there are many abiotic sources and sinks for O2, the presence of the profuse concentration of free oxygen in modern Earth's atmosphere and ocean is attributed to O2 production from the biological process of oxygenic photosynthesis in conjunction with a biological sink known as the biological pump and a geologic process of carbon burial involving plate tectonics.[8][9][10][7] Biology is the main driver of O2 flux on modern Earth, and the evolution of oxygenic photosynthesis by bacteria, which is discussed as part of The Great Oxygenation Event, is thought to be directly responsible for the conditions permitting the development and existence of all complex eukaryotic metabolism.[11][12][13]
Biological production
The main source of atmospheric free oxygen is photosynthesis, which produces sugars and free oxygen from carbon dioxide and water:
Photosynthesizing organisms include the plant life of the land areas as well as the phytoplankton of the oceans. The tiny marine cyanobacterium Prochlorococcus was discovered in 1986 and accounts for more than half of the photosynthesis of the open ocean.[14]
Abiotic production
An additional source of atmospheric free oxygen comes from photolysis, whereby high-energy ultraviolet radiation breaks down atmospheric water and nitrous oxide into component atoms. The free H and N atoms escape into space, leaving O2 in the atmosphere:
Biological consumption
The main way free oxygen is lost from the atmosphere is via respiration and decay, mechanisms in which animal life and bacteria consume oxygen and release carbon dioxide.
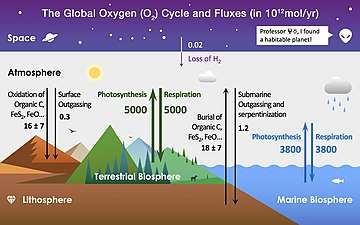
Capacities and fluxes
The following tables offer estimates of oxygen cycle reservoir capacities and fluxes. These numbers are based primarily on estimates from (Walker, J. C. G.):[9]
| Reservoir | Capacity (kg O2) |
Flux in/out (kg O2 per year) |
Residence time (years) |
|---|---|---|---|
| Atmosphere | 1.4×1018 | 3×1014 | 4500 |
| Biosphere | 1.6×1016 | 3×1014 | 50 |
| Lithosphere | 2.9×1020 | 6×1011 | 500000000 |
Table 2: Annual gain and loss of atmospheric oxygen (Units of 1010 kg O2 per year)[1]
| Photosynthesis (land) Photosynthesis (ocean) Photolysis of N2O Photolysis of H2O |
16,500 13,500 1.3 0.03 |
| Total gains | ~ 30,000 |
| Losses - respiration and decay | |
| Aerobic respiration Microbial oxidation Combustion of fossil fuel (anthropogenic) Photochemical oxidation Fixation of N2 by lightning Fixation of N2 by industry (anthropogenic) Oxidation of volcanic gases |
23,000 5,100 1,200 600 12 10 5 |
| Losses - weathering | |
| Chemical weathering Surface reaction of O3 |
50 12 |
| Total losses | ~ 30,000 |
Ozone
The presence of atmospheric oxygen has led to the formation of ozone (O3) and the ozone layer within the stratosphere:
The ozone layer is extremely important to modern life as it absorbs harmful ultraviolet radiation:
References
- Knoll AH, Canfield DE, Konhauser K (2012). "7". Fundamentals of geobiology. Chichester, West Sussex: John Wiley & Sons . pp. 93–104. ISBN 978-1-118-28087-4. OCLC 793103985.
- Petsch ST (2014). "The Global Oxygen Cycle". Treatise on Geochemistry. Elsevier. pp. 437–473. doi:10.1016/b978-0-08-095975-7.00811-1. ISBN 978-0-08-098300-4.
- Keeling RF, Shertz SR (August 1992). "Seasonal and interannual variations in atmospheric oxygen and implications for the global carbon cycle". Nature. 358 (6389): 723–727. Bibcode:1992Natur.358..723K. doi:10.1038/358723a0.
- Holland HD (2002). "Volcanic gases, black smokers, and the great oxidation event". Geochimica et Cosmochimica Acta. 66 (21): 3811–3826. Bibcode:2002GeCoA..66.3811H. doi:10.1016/S0016-7037(02)00950-X.
- Lasaga AC, Ohmoto H (2002). "The oxygen geochemical cycle: dynamics and stability". Geochimica et Cosmochimica Acta. 66 (3): 361–381. Bibcode:2002GeCoA..66..361L. doi:10.1016/S0016-7037(01)00685-8.
- Falkowski PG, Godfrey LV (August 2008). "Electrons, life and the evolution of Earth's oxygen cycle". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 363 (1504): 2705–16. doi:10.1098/rstb.2008.0054. PMC 2606772. PMID 18487127.
- Falkowski PG (January 2011). "The biological and geological contingencies for the rise of oxygen on Earth". Photosynthesis Research. 107 (1): 7–10. doi:10.1007/s11120-010-9602-4. PMID 21190137.
- Holland HD (June 2006). "The oxygenation of the atmosphere and oceans". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 361 (1470): 903–15. doi:10.1098/rstb.2006.1838. PMC 1578726. PMID 16754606.
- Walker JC (1980). "The Oxygen Cycle". The Natural Environment and the Biogeochemical Cycles. The Handbook of Environmental Chemistry. Springer Berlin Heidelberg. pp. 87–104. doi:10.1007/978-3-662-24940-6_5. ISBN 9783662229880.
- Sigman DM, Haug GH (December 2003). "The biological pump in the past.". Treatise on geochemistry. 6 (2nd ed.). p. 625. doi:10.1016/b978-0-08-095975-7.00618-5. ISBN 978-0-08-098300-4.
- Fischer WW, Hemp J, Johnson JE (June 2016). "Evolution of oxygenic photosynthesis". Annual Review of Earth and Planetary Sciences. 44 (1): 647–83. Bibcode:2016AREPS..44..647F. doi:10.1146/annurev-earth-060313-054810.
- Lyons TW, Reinhard CT, Planavsky NJ (February 2014). "The rise of oxygen in Earth's early ocean and atmosphere". Nature. 506 (7488): 307–15. Bibcode:2014Natur.506..307L. doi:10.1038/nature13068. PMID 24553238.
- Reinhard CT, Planavsky NJ, Olson SL, Lyons TW, Erwin DH (August 2016). "Earth's oxygen cycle and the evolution of animal life". Proceedings of the National Academy of Sciences of the United States of America. 113 (32): 8933–8. Bibcode:2016PNAS..113.8933R. doi:10.1073/pnas.1521544113. PMC 4987840. PMID 27457943.
- Nadis S (November 2003). "The Cells That Rule the Seas". Scientific American. 289 (6): 52–53. Bibcode:2003SciAm.289f..52N. doi:10.1038/scientificamerican1203-52. PMID 14631732.
Further reading
- Cloud P, Gibor A (September 1970). "The oxygen cycle". Scientific American. 223 (3): 110–123. Bibcode:1970SciAm.223c.110C. doi:10.1038/scientificamerican0970-110.
- Fasullo J. "Substitute Lectures for ATOC 3600". Principles of Climate, Lectures on the global oxygen cycle.
- Morris RM. "OXYSPHERE - A Beginners' Guide to the Biogeochemical Cycling of Atmospheric Oxygen". Archived from the original on 2004-11-03.