Oleic acid
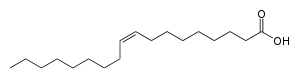
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega-9 fatty acid, abbreviated with a lipid number of 18:1 cis-9. It has the formula CH3(CH2)7CH=CH(CH2)7COOH.[2] The name derives from the Latin word oleum, which means oil.[3] It is the most common fatty acid in nature.[4] The salts and esters of oleic acid are called oleates.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(9Z)-Octadec-9-enoic acid | |
| Other names
Oleic acid (9Z)-Octadecenoic acid (Z)-Octadec-9-enoic acid cis-9-Octadecenoic acid cis-Δ9-Octadecenoic acid 18:1 cis-9 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.643 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H34O2 | |
| Molar mass | 282.468 g·mol−1 |
| Appearance | Pale yellow or brownish yellow oily liquid with lard-like odor |
| Density | 0.895 g/mL |
| Melting point | 13 to 14 °C (55 to 57 °F; 286 to 287 K) |
| Boiling point | 360 °C (680 °F; 633 K)[1] |
| Insoluble | |
| Solubility in Ethanol | Soluble |
| -208.5·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | JT Baker |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
Elaidic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
Fatty acids (or their salts) often do not occur as such in biological systems. Instead fatty acids such as oleic acid occur as their esters, commonly triglycerides, which are the greasy materials in many natural oils. Oleic acid is the most common monounsaturated fatty acid in nature. It is found in fats (triglycerides), the phospholipids that make membranes, cholesterol esters, and wax esters.[5]
Triglycerides of oleic acid comprise the majority of olive oil. Free oleic acid renders olive oil inedible.[6] It also makes up 59–75% of pecan oil,[7] 61% of canola oil,[8] 36–67% of peanut oil,[9] 60% of macadamia oil, 20–80% of sunflower oil,[10] 15–20% of grape seed oil, sea buckthorn oil, 40% of sesame oil,[2] and 14% of poppyseed oil. High oleic variants of plant sources such as sunflower (~80%) and canola oil (70%) also have been developed.[10] It also comprises 22.18% of the fats from the fruit of the durian species, Durio graveolens.[11] Karuka contains 52.39% oleic acid.[12] It is abundantly present in many animal fats, constituting 37 to 56% of chicken and turkey fat,[13] and 44 to 47% of lard.
Oleic acid is the most abundant fatty acid in human adipose tissue,[14] and second in abundance in human tissues overall, following palmitic acid.
Production and chemical behavior
The biosynthesis of oleic acid involves the action of the enzyme stearoyl-CoA 9-desaturase acting on stearoyl-CoA. In effect, stearic acid is dehydrogenated to give the monounsaturated derivative, oleic acid.[5]
Oleic acid undergoes the reactions of carboxylic acids and alkenes. It is soluble in aqueous base to give soaps called oleates. Iodine adds across the double bond. Hydrogenation of the double bond yields the saturated derivative stearic acid. Oxidation at the double bond occurs slowly in air, and is known as rancidification in foodstuffs and as drying in coatings. Reduction of the carboxylic acid group yields oleyl alcohol. Ozonolysis of oleic acid is an important route to azelaic acid. The coproduct is nonanoic acid:[15]
- H17C8CH=CHC7H14CO2H + 4"O" → H17C8CO2H + HO2CC7H14CO2H
Esters of azelaic acid find applications in lubrications and plasticizer.
Related compounds
The stereoisomer of oleic acid is called elaidic acid or trans-9-octadecenoic acid. These isomers have distinct physical properties and biochemical properties. Elaidic acid, the most abundant trans fatty acid in diet, appears to have an adverse effect on health.[16] A reaction that converts oleic acid to elaidic acid is called elaidinization.
Another naturally occurring isomer of oleic acid is petroselinic acid.
In chemical analysis, fatty acids are separated by gas chromatography of their methyl ester derivatives. Alternatively, separation of unsaturated isomers is possible by argentation thin-layer chromatography.[17]
In ethenolysis, methyl oleate, the methyl ester of the acid, converts to 1-decene and methyl 9-decenoate:[18]
- CH
3(CH
2)
7CH=CH(CH
2)
7CO
2Me + CH2=CH2 → CH3(CH2)7CH=CH2 + MeO2C(CH2)7CH=CH2
Uses
Oleic acid is as a component in many foods, in the form of its triglycerides. It is a component of the normal human diet, being a part of animal fats and vegetable oils.
Oleic acid as its sodium salt is a major component of soap as an emulsifying agent. It is also used as an emollient.[19] Small amounts of oleic acid are used as an excipient in pharmaceuticals, and it is used as an emulsifying or solubilizing agent in aerosol products.[20]
Niche uses
Oleic acid is used to induce lung damage in certain types of animals for the purpose of testing new drugs and other means to treat lung diseases. Specifically in sheep, intravenous administration of oleic acid causes acute lung injury with corresponding pulmonary edema.[21]
Oleic acid is used as a soldering flux in stained glass work for joining lead came.[22]
Oleic acid is widely used in the solution phase synthesis of nanoparticles, functioning as a kinetic knob to control the size and morphology of nanoparticles.[23]
Health effects
Oleic acid is a common monounsaturated fat in human diet. Monounsaturated fat consumption has been associated with decreased low-density lipoprotein (LDL) cholesterol, and possibly with increased high-density lipoprotein (HDL) cholesterol,[24] however, its ability to raise HDL is still debated. Presence of a ratio balancing the two types is considered essential for good health and that relationship remains subject to scientific debate as research continues.
Oleic acid may be responsible for the hypotensive (blood pressure reducing) effects of olive oil that is considered a health benefit.[25] Adverse effects have been documented in some research of oleic acid, however, since both oleic and monounsaturated fatty acid levels in the membranes of red blood cells have been associated with increased risk of breast cancer,[26] although other research indicates that the consumption of the oleate in olive oil has been associated with a decreased risk of breast cancer.[27]
FDA has approved a health claim on reduced risk of coronary heart disease for high oleic (> 70% oleic acid) oils.[28] Some oil plants have cultivars bred to increase the amount of oleic acid in the oils. In addition to providing a health claim, the heat stability and shelf life may also be improved, but only if the increase in monounsaturated oleic acid levels correspond to a substantial reduction in polyunsaturated fatty acid (especially α-Linolenic acid) content.[29] When the saturated fat or trans fat in a fried food is replaced with a stable high oleic oil, consumers may be able to avoid certain health risks associated with consuming saturated fat and trans fat.[30][31]
See also
- Elaidic acid – the corresponding trans isomer
- Oleylamine – the corresponding amine
- Oleamide – the corresponding amide
- Oleyl alcohol – the corresponding alcohol
References
- Young, Jay A. (2002). "Chemical Laboratory Information Profile: Oleic Acid". Journal of Chemical Education. 79 (1): 24. Bibcode:2002JChEd..79...24Y. doi:10.1021/ed079p24.
- Thomas, Alfred (2000). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_173. ISBN 978-3-527-30673-2.
- Bailey and Bailey, Dorothy and Kenneth (1929). "An Etymological Dictionary of Chemistry and Mineralogy". Nature. 124 (3134): 789–790. Bibcode:1929Natur.124..789V. doi:10.1038/124789b0.
- "9-Octadecenoic acid". PubChem, National Center for Biotechnology Information, US National Library of Medicine. 14 July 2018. Retrieved 19 July 2018.
- Ntambi, James M.; Miyazaki, Makoto (2003). "Recent insights into stearoyl-CoA desaturase-1". Current Opinion in Lipidology. 14 (3): 255–61. doi:10.1097/00041433-200306000-00005. PMID 12840656.CS1 maint: multiple names: authors list (link)
- "Olive Oil and Olive-Pomace Oil Grades and Standards | Agricultural Marketing Service". www.ams.usda.gov. Retrieved 2016-01-20.
- Villarreal-Lozoya, Jose E.; Lombardini, Leonardo; Cisneros-Zevallos, Luis (2007). "Phytochemical constituents and antioxidant capacity of different pecan Carya illinoinensis (Wangenh.) K. Koch] cultivars". Food Chemistry. 102 (4): 1241–1249. doi:10.1016/j.foodchem.2006.07.024.

- "Comparison of Dietary Fats Chart". Canola Council of Canada. Archived from the original on 2008-06-06. Retrieved 2008-09-03.
- Moore, K. M.; Knauft, D. A. (1989). "The Inheritance of High Oleic Acid in Peanut". The Journal of Heredity. 80 (3): 252–3. doi:10.1093/oxfordjournals.jhered.a110845.

- "Nutrient database, Release 25". United States Department of Agriculture.(NDB ID: 04678, 04584)
- Nasaruddin, Mohd hanif; Noor, Noor Qhairul Izzreen Mohd; Mamat, Hasmadi (2013). "Komposisi Proksimat dan Komponen Asid Lemak Durian Kuning (Durio graveolens) Sabah" [Proximate and Fatty Acid Composition of Sabah Yellow Durian (Durio graveolens)] (PDF). Sains Malaysiana (in Malay). 42 (9): 1283–1288. ISSN 0126-6039. OCLC 857479186. Retrieved 28 November 2017.
- Purwanto, Y.; Munawaroh, Esti (2010). "Etnobotani Jenis-Jenis Pandanaceae Sebagai Bahan Pangan di Indonesia" [Ethnobotany Types of Pandanaceae as Foodstuffs in Indonesia]. Berkala Penelitian Hayati (in Indonesian). 5A: 97–108. doi:10.5072/FK2/Z6P0OQ. ISSN 2337-389X. OCLC 981032990. Archived from the original (PDF) on 29 October 2018. Retrieved 25 October 2018.
- Nutter, Mary K.; Lockhart, Ernest E.; Harris, Robert S. (1943). "The chemical composition of depot fats in chickens and turkeys". Oil & Soap. 20 (11): 231–4. doi:10.1007/BF02630880.

- Kokatnur, MG; Oalmann, MC; Johnson, WD; Malcom, GT; Strong, JP (1979). "Fatty acid composition of human adipose tissue from two anatomical sites in a biracial community". The American Journal of Clinical Nutrition. 32 (11): 2198–205. doi:10.1093/ajcn/32.11.2198. PMID 495536.

- Cornils, Boy; Lappe, Peter (2000). "Dicarboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_523. ISBN 978-3-527-30673-2.
- Tardy, Anne-Laure; Morio, Beatrice; Chardigny, Jean-Michel; Malpuech-Brugere, Corinne (2011). "Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases". Nutrition Research Reviews. 24: 111–117. doi:10.1017/S0954422411000011. PMID 21320382.
- Breuer, B.; Fock, H. P. (1987). "Separation of fatty acids or methyl esters including positional and geometric isomers by alumina argentation thin-layer chromatography". J. Chromatogr. Sci. 25 (7): 302–306. doi:10.1093/chromsci/25.7.302. PMID 3611285.
- Marinescu, Smaranda C.; Schrock, Richard R.; Müller, Peter; Hoveyda, Amir H. (2009). "Ethenolysis Reactions Catalyzed by Imido Alkylidene Monoaryloxide Monopyrrolide (MAP) Complexes of Molybdenum". J. Am. Chem. Soc. 131 (31): 10840–10841. doi:10.1021/ja904786y. PMID 19618951.
- Carrasco, F. (2009). "Ingredientes Cosméticos". Diccionario de Ingredientes (4th ed.). p. 428. ISBN 978-84-613-4979-1.
- Smolinske, Susan C. (1992). Handbook of Food, Drug, and Cosmetic Excipients. pp. 247–8. ISBN 978-0-8493-3585-3.
- Julien, M; Hoeffel, JM; Flick, MR (1986). "Oleic acid lung injury in sheep". Journal of Applied Physiology. 60 (2): 433–40. doi:10.1152/jappl.1986.60.2.433. PMID 3949648.
- Duncan, Alastair (2003). The Technique of Leaded Glass. p. 77. ISBN 978-0-486-42607-5.
- Yin, Xi; Shi, Miao; Wu, Jianbo; Pan, Yung-Tin; Gray, Danielle L.; Bertke, Jeffery A.; Yang, Hong (11 September 2017). "Quantitative Analysis of Different Formation Modes of Platinum Nanocrystals Controlled by Ligand Chemistry". Nano Letters. 17 (10): 6146–6150. Bibcode:2017NanoL..17.6146Y. doi:10.1021/acs.nanolett.7b02751. PMID 28873317.
- "You Can Control Your Cholesterol: A Guide to Low-Cholesterol Living". Merck & Co. Inc. Archived from the original on 2009-03-03. Retrieved 2009-03-14.
- Teres, S.; Barcelo-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J. E.; Escriba, P. V. (2008). "Oleic acid content is responsible for the reduction in blood pressure induced by olive oil". Proceedings of the National Academy of Sciences. 105 (37): 13811–6. Bibcode:2008PNAS..10513811T. doi:10.1073/pnas.0807500105. JSTOR 25464133. PMC 2544536. PMID 18772370.
- Pala, V.; Krogh, V.; Muti, P.; Chajes, V.; Riboli, E.; Micheli, A.; Saadatian, M.; Sieri, S.; Berrino, F. (2001). "Erythrocyte Membrane Fatty Acids and Subsequent Breast Cancer: A Prospective Italian Study". JNCI Journal of the National Cancer Institute. 93 (14): 1088–95. doi:10.1093/jnci/93.14.1088. PMID 11459870.
- Martin-Moreno JM, Willett WC, Gorgojo L, Banegas JR, Rodriguez-Artalejo F, Fernandez-Rodriguez JC, Maisonneuve P, Boyle P (1994). "Dietary fat, olive oil intake and breast cancer risk". International Journal of Cancer. 58 (6): 774–780. doi:10.1002/ijc.2910580604. PMID 7927867.
- Nutrition, Center for Food Safety and Applied (20 December 2019). "FDA Completes Review of Qualified Health Claim Petition for Oleic Acid and the Risk of Coronary Heart Disease". FDA.
- Aladedunye, Felix; Przybylski, Roman (December 2013). "Frying stability of high oleic sunflower oils as affected by composition of tocopherol isomers and linoleic acid content". Food Chemistry. 141 (3): 2373–2378. doi:10.1016/j.foodchem.2013.05.061.
- "High-oleic canola oils and their food applications". The American Oil Chemists' Society.
- Trans Fat Task Force (June 2006). TRANSforming the Food Supply. ISBN 0-662-43689-X. Retrieved 7 January 2007.
External links
- FATTY ACIDS: STRAIGHT-CHAIN MONOENOIC (The AOCS Lipid Library)
- 9-octadecenoic acid (NIST Chemistry Webbook)
