Nitrosation
Nitrosation is a process of converting organic compounds into nitroso derivatives, i.e. compounds containing the R-NO functionality.
C-Nitroso compounds
C-Nitroso compounds, such as nitrosobenzene, are typically prepared by oxidation of hydroxylamines:
- RNHOH + [O] → RNO + H2O
S-Nitroso compounds
S-Nitroso compounds (S-nitrosothiols) are typically prepared by condensation of a thiol and nitrous acid:[1]
- RSH + HONO → RSNO + H2O
O-Nitroso compounds
O-Nitroso compounds are similar to S-nitroso compounds, but are less reactive because the oxygen atom is less nucleophilic than the sulfur atom. The formation of an alkyl nitrite from an alcohol and nitrous acid is a common example:
- ROH + HONO → RONO + H2O
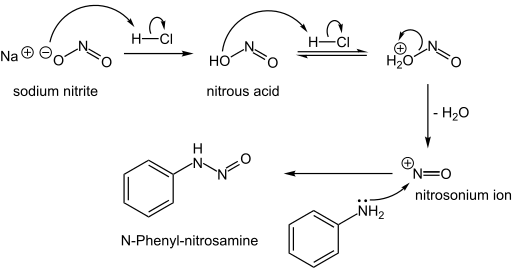
N-Nitrosamines
N-Nitrosamines, including the carcinogenic variety, arise from the reaction of nitrite sources with amino compounds, which can happen during the curing of meat. Typically this reaction proceeds via the attack of the nitrosonium electrophile on an amine:
- NO2− + 2 H+ → NO+ + H2O
- R2NH + NO+ → R2N-NO + H+
Formation of an N-nitrosamine:
References
- Wang, P. G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A. J. (2002). "Nitric Oxide Donors: Chemical Activities and Biological Applications". Chemical Reviews. 102 (4): 1091–1134. doi:10.1021/cr000040l. PMID 11942788.