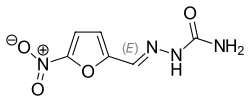
Nitrofurazone
Nitrofurazone (INN, trade name Furacin) is an antimicrobial organic compound belonging to the nitrofuran class.[1] It is most commonly used as a topical antibiotic ointment.[2] It is effective against gram-positive bacteria, gram-negative bacteria, and can be used in the treatment of trypanosomiasis.[1][3][4] Its use in medicine has become less frequent, as safer and more effective products have become available.[5] Nitrofurazone is listed under California Prop 65, and has demonstrated clear evidence to be mutagenic and carcinogenic during animal studies, and has been discontinued for human use in the USA.[2][5][6][7] The substance is pale yellow and crystalline. It was once widely used as an antibiotic for livestock.[8][9]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Aldomycin, Amifur, Chemfuran, Coxistat, Furacin, Furan-2, Furacinetten, Furaplast, Furazol W, Furesol Furracoccid, Mammex, Nefco, Nifuzon, Nitrofural, Vabrocid |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.403 |
| Chemical and physical data | |
| Formula | C6H6N4O4 |
| Molar mass | 198.138 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Medical uses
Human use
Nitrofurazone was previously available as a prescription in the U.S., and was indicated as a topical solution, topical cream, or topical ointment for the treatment of bacterial skin infections, wounds, burns, and ulcers.[2] It was also used as a prophylactic measure to prevent infection that could potentially result in skin graft rejection.[1][10] Nitrofurazone is still very popular as a topical solution for the treatment of tonsillitis in Russia.
Animal use
Nitrofurazone is indicated for topical use in dogs, cats, and horses, for the treatment or prophylactic treatment of superficial bacterial infections, burns, and cutaneous ulcers.[9] Preparations for treating infections, such as fin rot, in ornamental fish are also still commercially available.[11][12] The use of nitrofurazone, or related compounds, in animals raised for human consumption has been strictly banned.[11][13]
Pharmacokinetics
The mechanism of action is not fully understood, but nitrofurazone's antimicrobial properties are suspected to be due to the interference of DNA synthesis in the microorganism by inhibiting certain enzymes that are involved with glycolysis.[1][5] Other enzymes this may affect include, pyruvate dehydrogenase, citrate synthetase, malate dehydrogenase, glutathione reductase, and pyruvate decarboxylase.[1]
The metabolism of topically applied nitrofurazone is thought to be by 5-nitro reduction and cleavage of the -CH=N- linkage to generate a reactive species which can covalently bond to cellular macromolecules, none of the end products are thought to be antimicrobial.[1][14]
Adverse effects
Adverse effects for topical use are generally mild and include, erythema, pruritis, dermatitis, rash, edema or inflammation.[1][2]
Contraindications
People with chronic kidney disease and large total body surface area (TBSA) burns should not use nitrofurazone, as topical preparations commonly contain polyethylene glycol, which is readily absorbed through the skin. Rapid absorption of the medication induces increased serum osmolalities and anion gap, leading to death. [15] Symptoms are similar to ethylene glycol poisoning, in that increased serum calcium levels occur concurrently with decreased ionized calcium.[15]
Nitrofurans have been found in honey, meat and seafood. In people who have glucose-6-phosphate dehydrogenase deficiency (G-6-PD deficiency), ingestion of fish, poultry, or other foodstuff that has been treated with nitrofurans, will likely suffer from hemolysis of red blood cells as a result of eating the contaminated food.[5]
Toxicities
Nitrofurazone is suspected to be a human carcinogen and is included in California's list of toxic chemicals as defined by Proposition 65.[6][7][16] Studies demonstrate that nitrofurazone induces mammary tumors (fibroadenoma and adenocarcinoma) in rats and ovarian tumors in mice.[7] In addition, animal studies demonstrated an increased incidence in convulsive seizures, ovarian atrophy, testicular degeneration, and degeneration of articular cartilage.[7][17] Proper personal protective equipment should be utilized when handling nitrofurazone.[9]
References
- "Nitrofural". www.drugbank.ca. Retrieved 2018-03-14.
- "Nitrofurazone (Topical route)". Drugs.com.
- "Nitrofurazone". Pubchem. U.S. National Library of Medicine. Retrieved 2018-03-14.
- "nitrofurazone". www.glowm.com. Retrieved 2018-03-14.
- Perez S (26 May 2010). "Nitrofuran Analyses". Adpen Laboratories. Retrieved 14 March 2018.
- "Nitrofurazone". Office of Environmental Health Hazard Assessment. 2015-03-22. Retrieved 2018-03-14.
- National Toxicology Program (June 1988). NIH Publication No. 88-2593. "NTP Toxicology and Carcinogenesis Studies of Nitrofurazone in F344/N Rats and B6C3F1 Mice (Feed Studies)" (PDF). National Toxicology Program Technical Report Series. 337. National Institute of Health: 1–186.
- Vass M, Hruska K, Franek M (2008). "Nitrofuran antibiotics: a review on the application, prohibition and residual analysis". Veterinarni Medicina. 53: 469–500. doi:10.17221/1979-VETMED.
- "Nitrofurazone Ointment for Animal Use - Drugs.com". Drugs.com. Retrieved 2018-03-14.
- "Aldomycin". Minimally Invasive Neurosurgery Clinic. Retrieved 2018-03-14.
- Yanong RP (2017-01-05). "Use of Antibiotics in Ornamental Fish Aquaculture". edis.ifas.ufl.edu. Retrieved 2018-03-14.
- Giovanetti TA (1991). Discus Fish. Barron's Educational Series. ISBN 9780812046694.
- Center for Food Safety and Applied Nutrition, U.S. Department of Health and Human Services (April 2011). "Fish and Fishery Products Hazards and Controls Guidance" (PDF). www.fda.gov (Fourth ed.). Food and Drug Administration. pp. 183–208. Retrieved 2018-03-14.
- Residues of Some Veterinary Drugs in Animals and Foods: Monographs Prepared by the Fortieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, 9-18 June 1992. Joint FAO/WHO Expert Committee on Food Additives Meeting. Food and Agriculture Organization of the United Nations. 1993. ISBN 9789251032886.
- "Thermal Burns: Overview, Pathophysiology, Quantifying Burn Severity". 2017-12-29. Cite journal requires
|journal=(help) - Ryan A, Kaplan E, Laurieri N, Lowe E, Sim E (2011-08-12). "Activation of nitrofurazone by azoreductases: multiple activities in one enzyme". Scientific Reports. 1 (1): 63. Bibcode:2011NatSR...1E..63R. doi:10.1038/srep00063. PMC 3216550. PMID 22355582.
- "Abstract for TR-337". ntp.niehs.nih.gov. Retrieved 2018-03-14.