Myofibroblast
A myofibroblast is a cell that is in between a fibroblast and a smooth muscle cell in phenotype.
| Myofibroblast | |
|---|---|
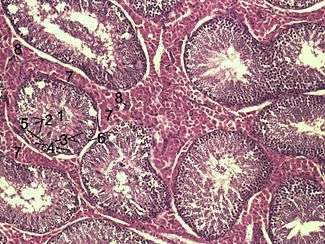 Histological section through testicular parenchyma of a boar. 1 Lumen of Tubulus seminiferus contortus, 2 spermatids, 3 spermatocytes, 4 spermatogonia, 5 Sertoli cell, 6 Myofibroblasts, 7 Leydig cells, 8 capillaries | |
| Details | |
| Identifiers | |
| Latin | myofibroblastus |
| MeSH | D058628 |
| TH | H2.00.03.0.01013 |
| Anatomical terms of microanatomy | |
Structure
Myofibroblasts are found subepithelially in many mucosal surfaces, for example, throughout almost the whole of the gastrointestinal and genitourinary tracts. Here they not only act as a regulator of the shape of the crypts and villi, but also act as stem-niche cells in the intestinal crypts and as parts of atypical antigen-presenting cells. They have both support as well as paracrine function in most places.
Location
They are found typically in granulation tissue and stroma of tumours. They generally line the gastrointestinal tract, where they regulate the shapes of crypts and villi.
Markers
Myofibroblasts usually stain for the intermediate filament vimentin which is a general mesenchymal marker and "alpha smooth muscle actin" (human gene = ACTA2) and for palladin, which is a cytoskeletal actin scaffold protein. They are positive for other smooth markers like intermediate filament type desmin in some tissues, but may be negative for desmin in some others. Similar heterogeneous positivity may exist for almost every smooth muscle marker except probably a few which are positive only in contractile smooth muscles like metavinculin and smoothelin.
Some myofibroblasts (especially if they have a stellate form) may also be positive for GFAP.
Development
There are many possible ways of myofibroblast development:
- Partial smooth muscle differentiation of a fibroblastic cell
- Activation of a stellate cell (e.g. hepatic Ito cells or pancreatic stellate cells).
- Loss of contractile phenotype (or acquisition of "synthetic phenotype") of a smooth muscle cell.
- Direct myofibroblastic differentiation of a progenitor cell resident in a stromal tissue.
- Homing and recruitment of a circulating mesenchymal precursor which can directly differentiate as above or indirectly differentiate through the other cell types as intermediates.
- Epithelial to mesenchymal transdifferentiation (EMT) of an epithelial cell.
Perhaps the most well studied pathway of myofibroblast formation, is TGF-beta1 dependent differentiation from fibroblast cells. Activation of the TGF-beta receptor 1 and TGF-beta receptor 2 leads to induction of the canonical SMAD2/SMAD3 pathway.[1] Together with the co-activation of the non-canonical EGFR pathway, these events lead to upregulation of the ACTA2 gene and subsequent alpha smooth muscle actin protein production. Several regulators of the myofibroblast differentiation pathway have been described, including hyaluronan and CD44 co-receptor activation of EGFR.[2]
Function
In many organs like liver, lung, and kidney they are primarily involved in fibrosis. In the wound tissue they are implicated in wound strengthening by extracellular collagen fiber deposition and then wound contraction by intracellular contraction and concomitant alignment of the collagen fibers by integrin-mediated pulling on to the collagen bundles. Pericytes and renal mesangial cells are some examples of modified myofibroblast-like cells.
Myofibroblasts may interfere with the propagation of electrical signals[3] controlling heart rhythm,[4] leading to arrhythmia in both patients who have suffered a heart attack and in foetuses. Ursodiol is a promising drug for this condition.[5]
Wound healing
Myofibroblasts can contract by using smooth muscle type actin-myosin complex, rich in a form of actin called alpha-smooth muscle actin. These cells are then capable of speeding wound repair by contracting the edges of the wound.
Early work on wound healing showed that granulation tissue taken from a wound could contract in vitro (or in an organ bath) in a similar fashion to smooth muscle, when exposed to substances that cause smooth muscle to contract, such as adrenaline or angiotensin.
More recently it has been shown that fibroblasts can transform into myofibroblasts with photobiomodulation.
After healing is complete, these cells are lost through apoptosis and it has been suggested that in several fibrotic diseases (for example liver cirrhosis, kidney fibrosis, retroperitoneal fibrosis) that this mechanism fails to work, leading to persistence of the myofibroblasts, and consequently expansion of the extracellular matrix (fibrosis) with contraction.
Similarly, in wounds that fail to resolve and become keloids or hypertrophic scars, myofibroblasts may persist, rather than disappearing by apoptosis.[6]
References
- Evans RA, Tian YC, Steadman R, Phillips AO (January 2003). "TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins". Experimental Cell Research. 282 (2): 90–100. doi:10.1016/S0014-4827(02)00015-0. PMID 12531695.
- Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, Steadman R (May 2013). "Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts". The Journal of Biological Chemistry. 288 (21): 14824–38. doi:10.1074/jbc.M113.451336. PMC 3663506. PMID 23589287.
- Quinn TA, Camelliti P, Rog-Zielinska EA, Siedlecka U, Poggioli T, O'Toole ET, Knöpfel T, Kohl P (December 2016). "Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics". Proceedings of the National Academy of Sciences of the United States of America. 113 (51): 14852–14857. doi:10.1073/pnas.1611184114. PMC 5187735. PMID 27930302.
- Gourdie RG, Dimmeler S, Kohl P (September 2016). "Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease". Nature Reviews. Drug Discovery. 15 (9): 620–38. doi:10.1038/nrd.2016.89. PMC 5152911. PMID 27339799.
- BBC News
- Frangogiannis NG (2017). "The extracellular matrix in myocardial injury, repair, and remodeling". J Clin Invest. 127 (5): 1600–1612. doi:10.1172/JCI87491. PMC 5409799. PMID 28459429.
External links
