Methylphosphonic acid
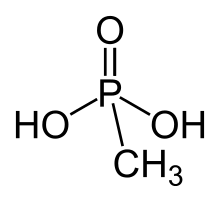
Methylphosphonic acid is an organophosphorus compound with the chemical formula. CH3P(O)(OH)2. The phosphorus center is tetrahedral and is bonded to a methyl group, two OH groups and an oxygen. Methylphosphonic acid is a white, non-volatile solid that is poorly soluble in organic solvent but soluble in water and common alcohols.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.370 |
| EC Number |
|
| KEGG | |
| MeSH | C032627 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH5O3P | |
| Molar mass | 96.02 |
| Appearance | White Solid |
| Melting point | 105 to 107 °C (221 to 225 °F; 378 to 380 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Methylphosphonic acid can be prepared via the Michaelis-Arbuzov reaction from triethylphosphite:[3]
- CH3Cl + P(OC2H5)3 → CH3PO(OC2H5)2 + C2H5Cl
The dialkylphosphonate is then treated with chlorotrimethylsilane:
- CH3PO(OC2H5)2 + 2 Me3SiCl → CH3PO(OSiMe3)2 + 2 C2H5Cl
The siloxyphosphinate is then hydrolyzed:
- CH3PO(OSiMe3)2 + 2H2O → CH3PO(OH)2 + 2 HOSiMe3
gollark: <@332271551481118732>> single player demo> emu war *online*
gollark: Completely* plausible.
gollark: Then, using highly advanced ontotechnology, the Emu Nation was sealed off from the rest of the world... and made into a computer game.
gollark: They took over half of the continent, and filled it with other animals to act as their workers and protectors.
gollark: Australia was overrun by the armies of genetically engineered emus in 2074.
References
- "Methylphosphonic Acid". Sigma-Aldrich. Retrieved 12 December 2013.
- "methylphosphonic acid - Compound Summary". NCBI. Retrieved 12 December 2013.
- Katritzky, Pilarski, Alan, Boguslaw. "A One-Pot Procedure For the Preparation of Phosphonic Acids From Alkyl Halides" (PDF). Archived from the original (PDF) on 23 September 2015. Retrieved 12 December 2013.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.