Mesylate
In chemistry, a mesylate is any salt or ester of methanesulfonic acid (CH3SO3H). In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate (as in imatinib mesilate, the mesylate salt of imatinib).[1]
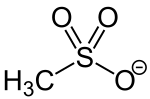
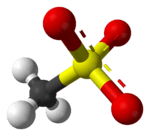
Mesylate esters are a group of organic compounds that share a common functional group with the general structure CH3SO2O–R, abbreviated MsO–R, where R is an organic substituent. Mesylate is considered a leaving group in nucleophilic substitution reactions.
Preparation
Mesylates are generally prepared by treating an alcohol and methanesulfonyl chloride in the presence of a base, such as triethylamine.[2]
Mesyl
Related to mesylate is the mesyl (Ms) or methanesulfonyl (CH3SO2) functional group. Methanesulfonyl chloride is often referred to as mesyl chloride. Whereas mesylates are often hydrolytically labile, mesyl groups, when attached to nitrogen, are resistant to hydrolysis.[3] This functional group appears in a variety of medications, particularly cardiac (antiarrhythmic) drugs, as a sulfonamide moiety. Examples include sotalol, ibutilide, sematilide, dronedarone, dofetilide, E-4031, and bitopertin.
Natural occurrence
Ice core samples from a single spot in Antarctica were found to have tiny inclusions of magnesium methanesulfonate dodecahydrate. This natural phase is recognized as the mineral ernstburkeite. It is extremely rare.[4][5]
See also
References
- World Health Organization (February 2006). "International Nonproprietary Names Modified" (PDF). INN Working Document 05.167/3. WHO. Cite journal requires
|journal=(help) Retrieved 5 December 2008. - Rick L. Danheiser; Yeun-Min Tsai; David M. Fink (1966). "A General Method for the Synthesis of Allenylsilanes: 1-Methyl-1-(trimethylsilyl)allene". Organic Syntheses. doi:10.15227/orgsyn.066.0001. (a procedure illustrating the use of mesylates).
- Valerie Vaillancourt, Michele M. Cudahy, Matthew M. Kreilein and Danielle L. Jacobs "Methanesulfonyl Chloride" in E-EROS Encyclopedia for Reagents in Organic Synthesis. doi:10.1002/047084289X.rm070.pub2
- Güner, Fatma Elif Genceli; Sakurai, Toshimitsu; Hondoh, Takeo (2013). "Ernstburkeite, Mg(CH3SO3)2·12H2O, a new mineral from Antarctica". European Journal of Mineralogy. 25 (1): 78–83. Bibcode:2013EJMin..25...78G. doi:10.1127/0935-1221/2013/0025-2257.
- Ernstburkeite, Mindat