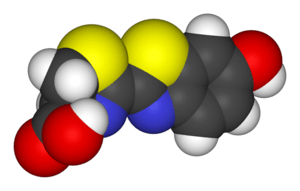
Luciferin
Luciferin (from the Latin lucifer, "light-bringer") is a generic term for the light-emitting compound found in organisms that generate bioluminescence. Luciferins typically undergo an enzyme-catalyzed reaction with molecular oxygen. The resulting transformation, which usually involves splitting off a molecular fragment, produces an excited state intermediate that emits light upon decaying to its ground state. The term may refer to molecules that are substrates for both luciferases and photoproteins.[1]
Types
Luciferins are a class of small-molecule substrates that react with oxygen in the presence of a luciferase (an enzyme) to release energy in the form of light. It is not known just how many types of luciferins there are, but some of the better-studied compounds are listed below.
Because of the chemical diversity of luciferins, there is no clear unifying mechanism of action, except that all require molecular oxygen,[2] which provides the needed energy.[3] The variety of luciferins and luciferases, their diverse reaction mechanisms and the scattered phylogenetic distribution indicate that many of them have arisen independently in the course of evolution.[2]
Firefly
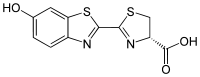
Firefly luciferin is the luciferin found in many Lampyridae species. It is the substrate of beetle luciferases (EC 1.13.12.7) responsible for the characteristic yellow light emission from fireflies, though can cross-react to produce light with related enzymes from non-luminous species.[4] The chemistry is unusual, as adenosine triphosphate (ATP) is required for light emission, in addition to molecular oxygen.[5]
Snail
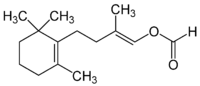
Latia luciferin is, in terms of chemistry, (E)-2-methyl-4-(2,6,6-trimethyl-1-cyclohex-1-yl)-1-buten-1-ol formate and is from the freshwater snail Latia neritoides.[6]
Bacterial
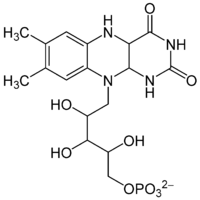
Bacterial luciferin is two-component system consisting of flavin mononucleotide and a fatty aldehyde found in bioluminescent bacteria.
Coelenterazine
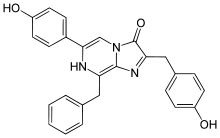
Coelenterazine is found in radiolarians, ctenophores, cnidarians, squid, brittle stars, copepods, chaetognaths, fish, and shrimp. It is the prosthetic group in the protein aequorin responsible for the blue light emission.[7]
Dinoflagellate

Dinoflagellate luciferin is a chlorophyll derivative (i. e. a tetrapyrrole) and is found in some dinoflagellates, which are often responsible for the phenomenon of nighttime glowing waves (historically this was called phosphorescence, but is a misleading term). A very similar type of luciferin is found in some types of euphausiid shrimp.[8]
Vargulin
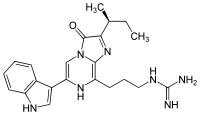
Vargulin is found in certain ostracods and deep-sea fish, to be specific, Poricthys. Like the compound coelenterazine, it is an imidazopyrazinone and emits primarily blue light in the animals.
Fungi
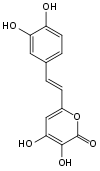
Foxfire is the bioluminescence created by some species of fungi present in decaying wood. While there may be multiple different luciferins within the kingdom of fungi, 3-hydroxy hispidin was determined to be the luciferin in the fruiting bodies of several species of fungi, including Neonothopanus nambi, Omphalotus olearius, Omphalotus nidiformis, and Panellus stipticus.[9]
References
- Hastings JW (1996). "Chemistries and colors of bioluminescent reactions: a review". Gene. 173 (1 Spec No): 5–11. doi:10.1016/0378-1119(95)00676-1. PMID 8707056.
- Hastings JW (1983). "Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems". J. Mol. Evol. 19 (5): 309–21. Bibcode:1983JMolE..19..309H. doi:10.1007/BF02101634. PMID 6358519.
- Schmidt-Rohr, K. (2020). "Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics” ACS Omega 5: 2221-2233. http://dx.doi.org/10.1021/acsomega.9b03352
- Viviani, V.R., Bechara, E.J.H. (1996). "Larval Tenebrio molitor (Coleoptera: Tenebrionidae) Fat Body Extracts Catalyze Firefly D-Luciferin-and ATP-Dependent Chemiluminescence: A Luciferase-like Enzyme". Photochemistry and Photobiology. 63 (6): 713–718. doi:10.1111/j.1751-1097.1996.tb09620.x.CS1 maint: multiple names: authors list (link)
- Green A, McElroy WD (October 1956). "Function of adenosine triphosphate in the activation of luciferin". Arch. Biochem. Biophys. 64 (2): 257–71. doi:10.1016/0003-9861(56)90268-5. PMID 13363432.
- EC 1.14.99.21. ORENZA: a database of ORphan ENZyme Activities, accessed 27 November 2009.
- Shimomura O, Johnson FH (April 1975). "Chemical nature of bioluminescence systems in coelenterates". Proc. Natl. Acad. Sci. U.S.A. 72 (4): 1546–49. Bibcode:1975PNAS...72.1546S. doi:10.1073/pnas.72.4.1546. PMC 432574. PMID 236561.
- Dunlap, JC; Hastings, JW; Shimomura, O (1980). "Crossreactivity between the light-emitting systems of distantly related organisms: Novel type of light-emitting compound". Proc. Natl. Acad. Sci. U.S.A. 77 (3): 1394–97. Bibcode:1980PNAS...77.1394D. doi:10.1073/pnas.77.3.1394. PMC 348501. PMID 16592787.
- Purtov KV, Petushkov VN, Baranov MS, Mineev KS, Rodionova NS, Kaskova ZM, Tsarkova AS, Petunin AI, Bondar VS, Rodicheva EK, Medvedeva SE, Oba Y, Arseniev AS, Lukyanov S, Gitelson JI, Yampolsky IV (2015). "The Chemical Basis of Fungal Bioluminescence". Angewandte Chemie International Edition. 54 (28): 8124–8128. doi:10.1002/anie.201501779. PMID 26094784.
External links
- "Major luciferin types". The Bioluminescence Web Page. University of California, Santa Barbara. 2009-01-09. Retrieved 2009-03-06.