Lefamulin
Lefamulin (tradename Xenleta) is an antibiotic used it to treat adults with community-acquired bacterial pneumonia.[1][2] It is taken by mouth or by injection into a vein.[1][2][3]
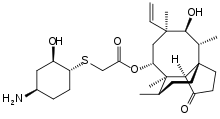 | |
| Clinical data | |
|---|---|
| Trade names | Xenleta |
| Other names | BC-3781 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Intravenous, by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C28H45NO5S |
| Molar mass | 507.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Relatively common side effects include diarrhea, nausea, pain at the site of injection, and liver inflammation.[1][4] It is a pleuromutilin antibiotic that inhibits the large subunit of bacterial ribosomes.[5]
Lefamulin was approved for medical use in the United States in 2019.[1] On 28 May 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Xenleta, intended for the treatment of community-acquired pneumonia (CAP) in adults.[6]
Medical uses
Lefamulin is used to treat adults with community-acquired bacterial pneumonia.[1][2] It was also investigated for treatment of acute bacterial skin and skin-structure infections (ABSSSI).[7]
Spectrum of activity
Lefamulin has in vitro activity against Streptococcus viridans, Moraxella catarrhalis, Enterococcus faecium, methicillin-resistant Staphylococcus aureus (MRSA), among other bacteria.[8][9]
History
It was developed by Nabriva Therapeutics and approved in the United States in 2019.[1] It was granted fast track status by the US Food and Drug Administration (FDA) in 2014. Although pleuromutilin antibiotics were first developed in the 1950s, lefamulin is the first to be used for systemic treatment of bacterial infections in humans.[10]
On 28 May 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Xenleta, intended for the treatment of community-acquired pneumonia (CAP) in adults.[6]
References
- "FDA approves new antibiotic to treat community-acquired bacterial pneumonia". U.S. Food and Drug Administration (FDA) (Press release). 19 August 2019. Archived from the original on 20 November 2019. Retrieved 19 November 2019.

- "Drug Trials Snapshots: Xenleta". U.S.Food and Drug Administration (FDA). 4 September 2019. Archived from the original on 20 November 2019. Retrieved 19 November 2019.
- File TM, Goldberg L, Das A, Sweeney C, Saviski J, Gelone SP, Seltzer E, Paukner S, Wicha WW, Talbot GH, Gasink LB (February 2019). "Efficacy and Safety of IV-to-Oral Lefamulin, a Pleuromutilin Antibiotic, for Treatment of Community-Acquired Bacterial Pneumonia: The Phase 3 LEAP 1 Trial". Clin. Infect. Dis. doi:10.1093/cid/ciz090. PMID 30722059.
- Alexander E, Goldberg L, Das AF, Moran GJ, Sandrock C, Gasink LB, Spera P, Sweeney C, Paukner S, Wicha WW, Gelone SP, Schranz J (September 2019). "Oral Lefamulin vs Moxifloxacin for Early Clinical Response Among Adults With Community-Acquired Bacterial Pneumonia: The LEAP 2 Randomized Clinical Trial". JAMA. doi:10.1001/jama.2019.15468. PMC 6865224. PMID 31560372.
- Andrei S, Droc G, Stefan G (December 2019). "FDA approved antibacterial drugs: 2018-2019" (PDF). Discoveries (Craiova). 7 (4): Article e102. doi:10.15190/d.2019.15. PMID 32309620.
- "Xenleta: Pending EC decision". European Medicines Agency (EMA). 29 May 2020. Retrieved 16 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Zeitlinger, M; Schwameis, R; Burian, A; Burian, B; Matzneller, P; Müller, M; Wicha, W. W.; Strickmann, D. B.; Prince, W (2016). "Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid". Journal of Antimicrobial Chemotherapy. 71 (4): 1022–6. doi:10.1093/jac/dkv442. PMID 26747098.
- H. Spreitzer (23 May 2016). "Neue Wirkstoffe - Lefamulin". Österreichische Apothekerzeitung (in German) (11/2016).
- Mendes, R. E.; Farrell, D. J.; Flamm, R. K.; Talbot, G. H.; Ivezic-Schoenfeld, Z; Paukner, S; Sader, H. S. (2016). "In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States". Antimicrobial Agents and Chemotherapy. 60: AAC.00627–16. doi:10.1128/AAC.00627-16. PMC 4914675. PMID 27161634.
- Veve, MP; Wagner, JL (September 2018). "Lefamulin: Review of a Promising Novel Pleuromutilin Antibiotic". Pharmacotherapy. 38 (9): 935–946. doi:10.1002/phar.2166. PMID 30019769.