Creatine
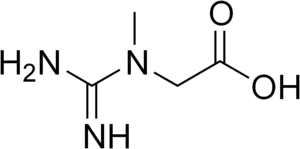
Creatine (/ˈkriːətiːn/ or /ˈkriːətɪn/)[1] is an organic compound with the nominal formula (H2N)(HN)CN(CH3)CH2CO2H. This species exists in various modifications (tautomers) in solution. Creatine is found in vertebrates where it facilitates recycling of adenosine triphosphate (ATP), the energy currency of the cell, primarily in muscle and brain tissue. Recycling is achieved by converting adenosine diphosphate (ADP) back to ATP via donation of phosphate groups. Creatine also acts as a buffer.[2]
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
2-[Carbamimidoyl(methyl)amino]acetic acid | |
| Other names
N-Carbamimidoyl-N-methylglycine; Methylguanidoacetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 907175 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.278 |
| EC Number |
|
| 240513 | |
| KEGG | |
| MeSH | Creatine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9N3O2 | |
| Molar mass | 131.135 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Melting point | 255 °C (491 °F; 528 K) |
| 13.3 g L−1 (at 18 °C) | |
| log P | −1.258 |
| Acidity (pKa) | 3.429 |
| Basicity (pKb) | 10.568 |
| Isoelectric point | 8.47 |
| Thermochemistry | |
Heat capacity (C) |
171.1 J K−1 mol−1 (at 23.2 °C) |
Std molar entropy (S |
189.5 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−538.06–−536.30 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−2.3239–−2.3223 MJ mol−1 |
| Pharmacology | |
| C01EB06 (WHO) | |
| Pharmacokinetics: | |
| 3 hours | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P305+351+338 | |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
Dimethylacetamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
Creatine was first identified in 1832 when Michel Eugène Chevreul isolated it from the basified water-extract of skeletal muscle. He later named the crystallized precipitate after the Greek word for meat, κρέας (kreas). In 1928, creatine was shown to exist in equilibrium with creatinine.[3] Studies in the 1920s showed that consumption of large amounts of creatine did not result in its excretion. This result pointed to the ability of the body to store creatine, which in turn suggested its use as a dietary supplement.[4]
In 1912, Harvard University researchers Otto Folin and Willey Glover Denis found evidence that ingesting creatine can dramatically boost the creatine content of the muscle.[5] In the late 1920s, after finding that the intramuscular stores of creatine can be increased by ingesting creatine in larger than normal amounts, scientists discovered creatine phosphate, and determined that creatine is a key player in the metabolism of skeletal muscle. The substance creatine is naturally formed in vertebrates.[6]
The discovery of phosphocreatine[7][8] was reported in 1927.[9][10][8] In the 1960s, creatine kinase (CK) was shown to phosphorylate ADP using phosphocreatine (PCr) to generate ATP. It follows that ATP, not PCr is directly consumed in muscle contraction. CK uses creatine to "buffer" the ATP/ADP ratio.[11]
While creatine's influence on physical performance has been well documented since the early twentieth century, it came into public view following the 1992 Olympics in Barcelona. An August 7, 1992 article in The Times reported that Linford Christie, the gold medal winner at 100 meters, had used creatine before the Olympics. An article in Bodybuilding Monthly named Sally Gunnell, who was the gold medalist in the 400-meter hurdles, as another creatine user. In addition, The Times also noted that 100 meter hurdler Colin Jackson began taking creatine before the Olympics.[12][13]
At the time, low-potency creatine supplements were available in Britain, but creatine supplements designed for strength enhancement were not commercially available until 1993 when a company called Experimental and Applied Sciences (EAS) introduced the compound to the sports nutrition market under the name Phosphagen.[14] Research performed thereafter demonstrated that the consumption of high glycemic carbohydrates in conjunction with creatine increases creatine muscle stores.[15]
Metabolic role
Creatine is a naturally occurring non-protein amino acid of which the primary metabolic role is to combine creatine with a phosphoryl group to generate phosphocreatine, which is used to regenerate ATP or Adenosine Triphosphate. Most of the human body's total creatine and phosphocreatine stores are found in skeletal muscle (95%), while the remainder is distributed in the blood, brain, testes, and other tissues.[16][17] The average amount of total creatine (creatine and phosphocreatine) stored in the body is approximately 120 mmol/kg of dry muscle mass.[18] However the upper limit of creatine storage post supplementation and dietary intervention is believed to be around 160 mmol/kg.[18] Studies have also shown that 1–2% of intramuscular creatine is degraded per day and an individual would need to consume about 1–3g of creatine per day to maintain average (unsupplemented) creatine storage.[18][19][20] For most individuals about half (1g/day) of this daily need is consumed from an omnivorous diet,[21][17] while the remaining amount is synthesized in the liver and kidneys.
Biosynthesis
Creatine synthesis primarily occurs in the liver and kidneys.[2][21] On average, it is produced endogenously at an estimated rate of about 8.3 mmol or 1 gram per day in young adults.[21][16]
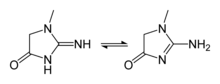
Creatine is not an essential nutrient[22] as it is naturally produced in the human body from the amino acids glycine and arginine, with an additional requirement for methionine to catalyze the transformation of guanidinoacetate to creatine. In the first step of the biosynthesis these two amino acids are combined by the enzyme arginine:glycine amidinotransferase (AGAT, EC:2.1.4.1) to form guanidinoacetate, which is then methylated by guanidinoacetate N-methyltransferase (GAMT, EC:2.1.1.2), using S-adenosyl methionine as the methyl donor. Creatine itself can be phosphorylated by creatine kinase to form phosphocreatine, which is used as an energy buffer in skeletal muscles and the brain. A cyclic form of creatine, called creatinine, exists in equilibrium with its tautomer and with creatine.
.png)
Phosphocreatine system
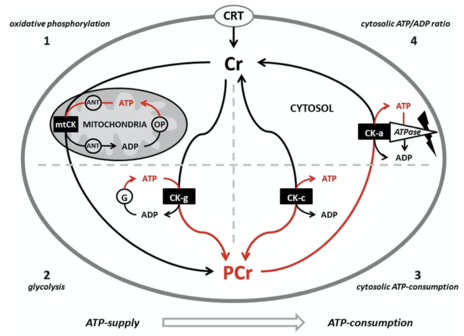
Creatine is transported through the blood and taken up by tissues with high energy demands, such as the brain and skeletal muscle, through an active transport system. The concentration of ATP in skeletal muscle is usually 2–5 mM, which would result in a muscle contraction of only a few seconds.[23] During times of increased energy demands, the phosphagen (or ATP/PCr) system rapidly resynthesizes ATP from ADP with the use of phosphocreatine (PCr) through a reversible reaction catalysed by the enzyme creatine kinase (CK). The phosphate group is attached to an NH center of the creatine. In skeletal muscle, PCr concentrations may reach 20–35 mM or more. Additionally, in most muscles, the ATP regeneration capacity of CK is very high and is therefore not a limiting factor. Although the cellular concentrations of ATP are small, changes are difficult to detect because ATP is continuously and efficiently replenished from the large pools of PCr and CK.[23] A proposed representation has been illustrated by Krieder et al.[24] Creatine has the ability to increase muscle stores of PCr, potentially increasing the muscle's ability to resynthesize ATP from ADP to meet increased energy demands.[25][26][27]
Creatine supplementation appears to increase the number of myonuclei that satellite cells will 'donate' to damaged muscle fibers, which increases the potential for growth of those fibers. This increase in myonuclei probably stems from creatine's ability to increase levels of the myogenic transcription factor MRF4.[28]
Genetic deficiencies
Genetic deficiencies in the creatine biosynthetic pathway lead to various severe neurological defects.[29] Clinically, there are three distinct disorders of creatine metabolism. Deficiencies in the two synthesis enzymes can cause L-arginine:glycine amidinotransferase deficiency caused by variants in GATM and guanidinoacetate methyltransferase deficiency, caused by variants in GAMT. Both biosynthetic defects are inherited in an autosomal recessive manner. A third defect, creatine transporter defect, is caused by mutations in SLC6A8 and inherited in a X-linked manner. This condition is related to the transport of creatine into the brain.[30]
Vegetarians
Some studies suggest that total muscle creatine is significantly lower in vegetarians than non-vegetarians.[31][32][30][17] This finding has been postulated to be due to an omnivorous diet being the primary source of creatine. Research shows that supplementation is needed to raise lacto-ovo vegetarian or vegan muscle creatine concentrations up to non-vegetarian levels.[31]
Pharmacokinetics
Most of the research to-date on creatine has predominantly focused on the pharmacological properties of creatine, yet there is a lack of research into the pharmacokinetics of creatine. Studies have not established pharmacokinetic parameters for clinical usage of creatine such as volume of distribution, clearance, bioavailability, mean residence time, absorption rate, and half life. A clear pharmacokinetic profile would need to be established prior to optimal clinical dosing.[33]
Dosing
Loading phase
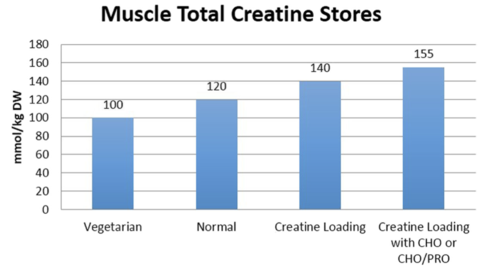
Taking creatine monohydrate in supplemental powder form showed that an initial loading dose of 5g creatine taken four times a day in equally spaced intervals (total of 20g/day) per day resulted in a rapid (20%) increase in total muscle creatine stores after 6 days.[18] Alternatively, an approximation of 0.3g/kg/day divided into 4 equal spaced intervals has also been suggested since creatine needs may vary based on body weight.[24][18] It has also been shown that taking a lower dose of 3g four times a day for a total of 12g/day for 28 days can also increase total muscle creatine storage to the same amount as the rapid loading dose of 20g/day for 6 days.[18] However, a 28 day loading phase does not allow for ergogenic benefits of creatine supplementation to be realized until fully saturated muscle storage.
Supplementing creatine with carbohydrates or carbohydrates and protein has been shown to augment creatine retention.[34][15]
This elevation in muscle creatine storage has been correlated with ergogenic benefits discussed in the therapeutic uses section. However, higher doses for longer periods of time are being studied to offset creatine synthesis deficiencies and mitigating diseases.[35][36][30]
Maintenance phase
After the 5–7 day loading phase, muscle creatine stores are fully saturated and supplementation only needs to cover the amount of creatine broken down per day. This maintenance dose was originally reported to be around 2–3g/day (or 0.03g/kg/day),[18] however, recent studies have suggested 3–5g/day maintenance dose to maintain saturated muscle creatine.[15][20][37][38]
Absorption
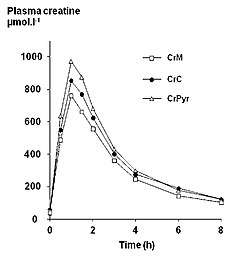
Endogenous serum or plasma creatine concentrations in healthy adults are normally in a range of 2–12 mg/L. A single 5 g (5000 mg) oral dose in healthy adults results in a peak plasma creatine level of approximately 120 mg/L at 1–2 hours post-ingestion. Creatine has a fairly short elimination half life, averaging just less than 3 hours, so to maintain an elevated plasma level it would be necessary to take small oral doses every 3–6 hours throughout the day.
Therapeutic usage
Creatine supplements are marketed in ethyl ester, gluconate, monohydrate, and nitrate forms.[41]

Exercise performance
Creatine as a performance enhancing supplement has received support from the Journal of the International Society of Sports Nutrition,[42] and in joint position stands from the American College of Sports Medicine, Academy of Nutrition and Dietetics, and Dietitians in Canada.[43]

Potential Creatine use can increase maximum power and performance in high-intensity anaerobic repetitive work (periods of work and rest) by 5 to 15%.[44][45][46] Creatine has no significant effect on aerobic endurance, though it will increase power during short sessions of high-intensity aerobic exercise.[47][48]
A survey of 21,000 college athletes showed that 14% of athletes take creatine supplements to improve performance.[49] Non-athletes report taking creatine supplements to improve appearance.[49]
Creatine is reported to increase cognitive performance,[50] especially in individuals with inadequate intakes in their diet and is claimed by some sources[51][52] to be a nootropic supplement.
Preventative health
A clinical study has shown that the intake of pure, high-quality creatine alone, or in combination with exercise, may reduce and delay age-related muscle atrophy, by improving fat-free body mass, muscle strength and endurance, while simultaneously improving bone density.[53]
Muscular disease
A meta-analysis found that creatine treatment increased muscle strength in muscular dystrophies, and potentially improved functional performance.[54] Creatine treatment does not appear to improve muscle strength in people who have metabolic myopathies.[54] High doses of creatine lead to increased muscle pain and an impairment in activities of daily living when taken by people who have McArdle disease.[54]
According to a clinical study focusing on people with various muscular dystrophies, using a pure form of creatine monohydrate can be beneficial in rehabilitation after injuries and immobilization.[55]
Mitochondrial diseases
Parkinson's disease
Creatine's impact on mitochondrial function has led to research on its efficacy and safety for slowing Parkinson's disease. As of 2014, the evidence did not provide a reliable foundation for treatment decisions, due to risk of bias, small sample sizes, and the short duration of trials.[56]
Huntington's disease
Several primary studies[57][58][59] have been completed but no systematic review on Huntington's disease has been completed yet.
ALS
It is ineffective as a treatment for amyotrophic lateral sclerosis.[60]
Adverse effects
- Weight gain due to extra water retention to the muscle
- Potential muscle cramps / strains / pulls
- Upset stomach
- Diarrhea
- Dizziness
- High blood pressure due to extra water consumption
One well-documented effect of creatine supplementation is weight gain within the first week of the supplement schedule, likely attributable to greater water retention due to the increased muscle creatine concentrations.[63]
A 2009 systematic review discredited concerns that creatine supplementation could affect hydration status and heat tolerance and lead to muscle cramping and diarrhea.[64][65]
Renal function
A 2019 systematic review published by the National Kidney Foundation investigated whether creatine supplementation had adverse effects on renal function.[66] They identified 15 studies from 1997 - 2013 that looked at standard creatine loading and maintenance protocols of 4-20g/day of creatine versus placebo. They utilized serum creatinine, creatinine clearance, and serum urea levels as a measure of renal damage. While in general creatine supplementation resulted in slightly elevated creatinine levels that remained within normal limits, supplementation did not induce renal damage (P value< 0.001). Special populations included in the 2019 Systematic review included type 2 diabetic patients[67] and post-menopausal women,[68] bodybuilders,[69] athletes,[70] and resistance trained populations.[71][72][73] The study also discussed 3 case studies where there were reports that creatine affected renal function.[74][75][76]
In a joint statement between the American College of Sports Medicine, Academy of Nutrition and Dietetics, and Dietitians in Canada on performance enhancing nutrition strategies, creatine was included in their list of ergogenic aids and they do not list renal function as a concern for use.[43]
The most recent position stand on creatine from the Journal of International Society of Sports Nutrition states that creatine is safe to take in health populations from infants to the elderly to performance athletes. They also state that long term (5 years) use of creatine has been considered safe.[24].
At this occasion, it is important to mention that kidneys themselves, for normal physiological function, need Phospho-Creatine and Creatine and indeed kidneys express significant amounts of Creatine Kinases (BB-CK and u-mtCK isoenzymes)[77]. At the same time, the first of two steps for endogenous Creatine synthesis takes place in the kidneys itself. Patients with kidney disease and those undergoing dialysis treatment generally show significantly lowe levels of Creatine in their organs, since the pathological kidney are 1) hampered in creatine synthesis capability and 2) in back-resoption of Creatine from the urine in the distal tubules. In addition, dialysis patients are losing Creatine due to wash out by the very dialysis treatment and thus become chronically Creatine-depleted. This situation is ascerbated by tha fact that dialysis patients generally consume less meat and fish, the alimentary sources of Creatine. Therefore, to alleviate chronic Creatine depletion in these patients and allow to refill-up the organ pools of Creatine, it was recently proposed to supplement dialysis patients with extra Creatine, preferably by intra-dialytic Creatine supplementation. Such a supplementation with Creatine of dialysis patients is expected to significantly improve the health and quality of the patients by improving muscle strengh, coordination of movement, brain function and to aleviate depressions and chronic fatigue that are common in this patients. [78].
Safety
Contamination
A 2011 survey of 33 supplements commercially available in Italy found that over 50% of them exceeded the European Food Safety Authority recommendations in at least one contaminant. The most prevalent of these contaminants was creatinine, a breakdown product of creatine also produced by the body.[79] Creatinine was present in higher concentrations than the European Food Safety Authority recommendations in 44% of the samples. About 15% of the samples had detectable levels of dihydro-1,3,5-triazine or a high dicyandiamide concentration. Heavy metals contamination was not found to be a concern, with only minor levels of mercury being detectable. Two studies reviewed in 2007 found no impurities.[80]
Interactions
Creatine taken with medications that can harm the kidney can increase the risk of kidney damage:[81]
- Nonsteroidal anti-inflammatory drugs (NSAIDs) – some examples are ibuprofen (Motrin, Advil) and naproxen (Aleve)
- Diuretics (water pills) – an example is furosemide (Lasix)
- Cimetidine (Tagamet)
- Probenicid
A National Institutes of Health study suggests that caffeine interacts with creatine to increase the rate of progression of Parkinson's Disease.[82]
Food and cooking
When creatine is mixed with protein and sugar at high temperatures (above 148 °C), the resulting reaction produces carcinogenic heterocyclic amines (HCAs).[83] Such a reaction happens when grilling or pan-frying meat.[84] Creatine content (as a percentage of crude protein) can be used as an indicator of meat quality.[85]
Dietary considerations
Creatine-monohydrate is suitable for vegetarians and vegans, as the raw materials used for the production of the supplement have no animal origin.[86]
See also
References
- Stout JR, Antonio J, Kalman E, eds. (2008). Essentials of Creatine in Sports and Health. Humana. ISBN 978-1-59745-573-2.
- Barcelos RP, Stefanello ST, Mauriz JL, Gonzalez-Gallego J, Soares FA (2016). "Creatine and the Liver: Metabolism and Possible Interactions". Mini Reviews in Medicinal Chemistry. 16 (1): 12–8. doi:10.2174/1389557515666150722102613. PMID 26202197.
The process of creatine synthesis occurs in two steps, catalyzed by L-arginine:glycine amidinotransferase (AGAT) and guanidinoacetate N-methyltransferase (GAMT), which take place mainly in kidney and liver, respectively. This molecule plays an important energy/pH buffer function in tissues, and to guarantee the maintenance of its total body pool, the lost creatine must be replaced from diet or de novo synthesis.
- Cannan RK, Shore A (1928). "The creatine-creatinine equilibrium. The apparent dissociation constants of creatine and creatinine". The Biochemical Journal. 22 (4): 920–9. doi:10.1042/bj0220920. PMC 1252207. PMID 16744118.
- Volek JS, Ballard KD, Forsythe CE (2008). "Overview of Creatine Metabolism". In Stout JR, Antonio J, Kalman E (eds.). Essentials of Creatine in Sports and Health. Humana. pp. 1–23. ISBN 978-1-59745-573-2.
- Folin O, Denis W (1912). "Protein metabolism from the standpoint of blood and tissue analysis". Journal of Biological Chemistry. 12 (1): 141–61.
- Brosnan JT, da Silva RP, Brosnan ME (May 2011). "The metabolic burden of creatine synthesis". Amino Acids. 40 (5): 1325–31. doi:10.1007/s00726-011-0853-y. PMID 21387089.
- Saks V (2007). Molecular system bioenergetics: energy for life. Weinheim: Wiley-VCH. p. 2. ISBN 978-3-527-31787-5.
- Ochoa S (1989). Sherman EJ, National Academy of Sciences (eds.). David Nachmansohn. Biographical Memoirs. 58. National Academies Press. pp. 357–404. ISBN 978-0-309-03938-3.
- Eggleton P, Eggleton GP (1927). "The Inorganic Phosphate and a Labile Form of Organic Phosphate in the Gastrocnemius of the Frog". The Biochemical Journal. 21 (1): 190–5. doi:10.1042/bj0210190. PMC 1251888. PMID 16743804.
- Fiske CH, Subbarow Y (April 1927). "The nature of the 'inorganic phosphate' in voluntary muscle". Science. 65 (1686): 401–3. Bibcode:1927Sci....65..401F. doi:10.1126/science.65.1686.401. PMID 17807679.
- Wallimann T (2007). "Introduction – Creatine: Cheap Ergogenic Supplement with Great Potential for Health and Disease". In Salomons GS, Wyss M (eds.). Creatine and Creatine Kinase in Health and Disease. Springer. pp. 1–16. ISBN 978-1-4020-6486-9.
- "Supplement muscles in on the market". National Review of Medicine. 30 July 2004. Archived from the original on 16 November 2006. Retrieved 25 May 2011.
- Passwater RA (2005). Creatine. p. 9. ISBN 978-0-87983-868-3. Retrieved 25 May 2011.
- Stoppani J (May 2004). Creatine new and improved: recent high-tech advances have made creatine even more powerful. Here's how you can take full advantage of this super supplement. Muscle & Fitness. Retrieved 29 March 2010.
- Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL (November 1996). "Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans". The American Journal of Physiology. 271 (5 Pt 1): E821-6. doi:10.1152/ajpendo.1996.271.5.E821. PMID 8944667.
- Cooper R, Naclerio F, Allgrove J, Jimenez A (July 2012). "Creatine supplementation with specific view to exercise/sports performance: an update". Journal of the International Society of Sports Nutrition. 9 (1): 33. doi:10.1186/1550-2783-9-33. PMC 3407788. PMID 22817979.
Creatine is produced endogenously at an amount of about 1 g/d. Synthesis predominately occurs in the liver, kidneys, and to a lesser extent in the pancreas. The remainder of the creatine available to the body is obtained through the diet at about 1 g/d for an omnivorous diet. 95% of the bodies creatine stores are found in the skeletal muscle and the remaining 5% is distributed in the brain, liver, kidney, and testes [1].
- Brosnan ME, Brosnan JT (August 2016). "The role of dietary creatine". Amino Acids. 48 (8): 1785–91. doi:10.1007/s00726-016-2188-1. PMID 26874700.
The daily requirement of a 70-kg male for creatine is about 2 g; up to half of this may be obtained from a typical omnivorous diet, with the remainder being synthesized in the body ... More than 90% of the body’s creatine and phosphocreatine is present in muscle (Brosnan and Brosnan 2007), with some of the remainder being found in the brain (Braissant et al. 2011). ... Creatine synthesized in liver must be secreted into the bloodstream by an unknown mechanism (Da Silva et al. 2014a)
- Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL (July 1996). "Muscle creatine loading in men". Journal of Applied Physiology. 81 (1): 232–7. doi:10.1152/jappl.1996.81.1.232. PMID 8828669.
- Balsom PD, Söderlund K, Ekblom B (October 1994). "Creatine in humans with special reference to creatine supplementation". Sports Medicine. 18 (4): 268–80. doi:10.2165/00007256-199418040-00005. PMID 7817065.
- Harris RC, Söderlund K, Hultman E (September 1992). "Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation". Clinical Science. 83 (3): 367–74. doi:10.1042/cs0830367. PMID 1327657.
- Brosnan JT, da Silva RP, Brosnan ME (May 2011). "The metabolic burden of creatine synthesis". Amino Acids. 40 (5): 1325–31. doi:10.1007/s00726-011-0853-y. PMID 21387089.
Creatinine loss averages approximately 2 g (14.6 mmol) for 70 kg males in the 20- to 39-year age group. ... Table 1 Comparison of rates of creatine synthesis in young adults with dietary intakes of the three precursor amino acids and with the whole body transmethylation flux
Creatine synthesis (mmol/day) 8.3 - "Creatine". Beth Israel Deaconess Medical Center. Retrieved 23 August 2010.
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (January 1992). "Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis". The Biochemical Journal. 281 ( Pt 1) (Pt 1): 21–40. doi:10.1042/bj2810021. PMC 1130636. PMID 1731757.
- Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. (2017). "International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine". Journal of the International Society of Sports Nutrition. 14: 18. doi:10.1186/s12970-017-0173-z. PMC 5469049. PMID 28615996.
- Spillane M, Schoch R, Cooke M, Harvey T, Greenwood M, Kreider R, Willoughby DS (February 2009). "The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levels". Journal of the International Society of Sports Nutrition. 6 (1): 6. doi:10.1186/1550-2783-6-6. PMC 2649889. PMID 19228401.
- Wallimann T, Tokarska-Schlattner M, Schlattner U (May 2011). "The creatine kinase system and pleiotropic effects of creatine". Amino Acids. 40 (5): 1271–96. doi:10.1007/s00726-011-0877-3. PMC 3080659. PMID 21448658..
- T. Wallimann, M. Tokarska-Schlattner, D. Neumann u. a.: The Phosphocreatine Circuit: Molecular and Cellular Physiology of Creatine Kinases, Sensitivity to Free Radicals, and Enhancement by Creatine Supplementation. In: Molecular System Bioenergetics: Energy for Life. 22. November 2007. doi:10.1002/9783527621095.ch7C
- Hespel P, Eijnde BO, Derave W, Richter EA (2001). "Creatine supplementation: exploring the role of the creatine kinase/phosphocreatine system in human muscle". Canadian Journal of Applied Physiology. 26 Suppl: S79-102. doi:10.1139/h2001-045. PMID 11897886.
- "L-Arginine:Glycine Amidinotransferase". Retrieved 16 August 2010.
- Braissant O, Henry H, Béard E, Uldry J (May 2011). "Creatine deficiency syndromes and the importance of creatine synthesis in the brain" (PDF). Amino Acids. 40 (5): 1315–24. doi:10.1007/s00726-011-0852-z. PMID 21390529.
- Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnopolsky M (November 2003). "Effect of creatine and weight training on muscle creatine and performance in vegetarians". Medicine and Science in Sports and Exercise. 35 (11): 1946–55. doi:10.1249/01.MSS.0000093614.17517.79. PMID 14600563.
- Benton D, Donohoe R (April 2011). "The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores". The British Journal of Nutrition. 105 (7): 1100–5. doi:10.1017/S0007114510004733. PMID 21118604.
- Persky AM, Brazeau GA (June 2001). "Clinical pharmacology of the dietary supplement creatine monohydrate". Pharmacological Reviews. 53 (2): 161–76. PMID 11356982.
- Steenge GR, Simpson EJ, Greenhaff PL (September 2000). "Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans". Journal of Applied Physiology. 89 (3): 1165–71. doi:10.1152/jappl.2000.89.3.1165. PMID 10956365.
- Hanna-El-Daher L, Braissant O (August 2016). "Creatine synthesis and exchanges between brain cells: What can be learned from human creatine deficiencies and various experimental models?". Amino Acids. 48 (8): 1877–95. doi:10.1007/s00726-016-2189-0. PMID 26861125.
- Bender A, Klopstock T (August 2016). "Creatine for neuroprotection in neurodegenerative disease: end of story?". Amino Acids. 48 (8): 1929–40. doi:10.1007/s00726-015-2165-0. PMID 26748651.
- Kreider RB (February 2003). "Effects of creatine supplementation on performance and training adaptations". Molecular and Cellular Biochemistry. 244 (1–2): 89–94. doi:10.1023/A:1022465203458. PMID 12701815.
- Greenhaff PL, Casey A, Short AH, Harris R, Soderlund K, Hultman E (May 1993). "Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man". Clinical Science. 84 (5): 565–71. doi:10.1042/cs0840565. PMID 8504634.
- Jäger R, Harris RC, Purpura M, Francaux M (November 2007). "Comparison of new forms of creatine in raising plasma creatine levels". Journal of the International Society of Sports Nutrition. 4: 17. doi:10.1186/1550-2783-4-17. PMC 2206055. PMID 17997838.
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P (December 1997). "Long-term creatine intake is beneficial to muscle performance during resistance training". Journal of Applied Physiology. 83 (6): 2055–63. doi:10.1152/jappl.1997.83.6.2055. PMID 9390981.
- Cooper R, Naclerio F, Allgrove J, Jimenez A (July 2012). "Creatine supplementation with specific view to exercise/sports performance: an update". Journal of the International Society of Sports Nutrition. 9 (1): 33. doi:10.1186/1550-2783-9-33. PMC 3407788. PMID 22817979.
- Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, et al. (August 2018). "ISSN exercise & sports nutrition review update: research & recommendations". Journal of the International Society of Sports Nutrition. 15 (1): 38. doi:10.1186/s12970-018-0242-y. PMC 6090881. PMID 30068354.
- Rodriguez NR, Di Marco NM, Langley S (March 2009). "American College of Sports Medicine position stand. Nutrition and athletic performance". Medicine and Science in Sports and Exercise. 41 (3): 709–31. doi:10.1249/MSS.0b013e31890eb86. PMID 19225360.
- Bemben MG, Lamont HS (2005). "Creatine supplementation and exercise performance: recent findings". Sports Medicine. 35 (2): 107–25. doi:10.2165/00007256-200535020-00002. PMID 15707376.
- Bird SP (December 2003). "Creatine supplementation and exercise performance: a brief review". Journal of Sports Science & Medicine. 2 (4): 123–32. PMC 3963244. PMID 24688272.
- Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage FX, Dutheil F (September 2015). "Creatine Supplementation and Lower Limb Strength Performance: A Systematic Review and Meta-Analyses". Sports Medicine. 45 (9): 1285–1294. doi:10.1007/s40279-015-0337-4. PMID 25946994.
- Engelhardt M, Neumann G, Berbalk A, Reuter I (July 1998). "Creatine supplementation in endurance sports". Medicine and Science in Sports and Exercise. 30 (7): 1123–9. doi:10.1097/00005768-199807000-00016. PMID 9662683.
- Graham AS, Hatton RC (1999). "Creatine: a review of efficacy and safety". Journal of the American Pharmaceutical Association. 39 (6): 803–10, quiz 875–7. doi:10.1016/s1086-5802(15)30371-5. PMID 10609446.
- "Office of Dietary Supplements - Dietary Supplements for Exercise and Athletic Performance". Retrieved 5 May 2018.
- Ling J, Kritikos M, Tiplady B (December 2009). "Cognitive effects of creatine ethyl ester supplementation" (PDF). Behavioural Pharmacology. 20 (8): 673–9. doi:10.1097/FBP.0b013e3283323c2a. PMID 19773644.
- "Creatine – Nootropics Expert".
- "8 Reasons Creatine is the Best Nootropic Source Right Now". 30 October 2018.
- Wallimann T (2014). "Positive Wirkung von Kreatin im Alter und für Rehabilitation" (PDF). Schweizer Zeitschrift für Ernährungsmedizin (2): 31–33. ISSN 1660-4695.
- Kley RA, Tarnopolsky MA, Vorgerd M (June 2013). "Creatine for treating muscle disorders". The Cochrane Database of Systematic Reviews (6): CD004760. doi:10.1002/14651858.CD004760.pub4. PMC 6492334. PMID 23740606.
- Walter MC, Lochmüller H, Reilich P, Klopstock T, Huber R, Hartard M, et al. (May 2000). "Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study". Neurology. 54 (9): 1848–50. doi:10.1212/wnl.54.9.1848. PMID 10802796.
- Xiao Y, Luo M, Luo H, Wang J (June 2014). "Creatine for Parkinson's disease". The Cochrane Database of Systematic Reviews (6): CD009646. doi:10.1002/14651858.cd009646.pub2. PMID 24934384.
- Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, et al. (October 2003). "Creatine supplementation in Huntington's disease: a placebo-controlled pilot trial". Neurology. 61 (7): 925–30. doi:10.1212/01.wnl.0000090629.40891.4b. PMID 14557561.
- Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, et al. (January 2005). "Creatine supplementation lowers brain glutamate levels in Huntington's disease". Journal of Neurology. 252 (1): 36–41. doi:10.1007/s00415-005-0595-4. PMID 15672208.
- Hersch SM, Schifitto G, Oakes D, Bredlau AL, Meyers CM, Nahin R, Rosas HD (August 2017). "The CREST-E study of creatine for Huntington disease: A randomized controlled trial". Neurology. 89 (6): 594–601. doi:10.1212/WNL.0000000000004209. PMC 5562960. PMID 28701493.
- Pastula DM, Moore DH, Bedlack RS (December 2012). "Creatine for amyotrophic lateral sclerosis/motor neuron disease". The Cochrane Database of Systematic Reviews. 12: CD005225. doi:10.1002/14651858.CD005225.pub3. PMID 23235621.
- Francaux M, Poortmans JR (December 2006). "Side effects of creatine supplementation in athletes". International Journal of Sports Physiology and Performance. 1 (4): 311–23. doi:10.1123/ijspp.1.4.311. PMID 19124889. S2CID 21330062.
- Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, et al. (August 2007). "International Society of Sports Nutrition position stand: creatine supplementation and exercise". Journal of the International Society of Sports Nutrition. jissn. 4: 6. doi:10.1186/1550-2783-4-6. PMC 2048496. PMID 17908288.
- Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. (13 June 2017). "International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine". Journal of the International Society of Sports Nutrition. 14: 18. doi:10.1186/s12970-017-0173-z. PMC 5469049. PMID 28615996.
- Lopez RM, Casa DJ, McDermott BP, Ganio MS, Armstrong LE, Maresh CM (2009). "Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses". Journal of Athletic Training. 44 (2): 215–23. doi:10.4085/1062-6050-44.2.215. PMC 2657025. PMID 19295968.
- Dalbo VJ, Roberts MD, Stout JR, Kerksick CM (July 2008). "Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration". British Journal of Sports Medicine. 42 (7): 567–73. doi:10.1136/bjsm.2007.042473. PMID 18184753.
- de Souza E, Silva A, Pertille A, Reis Barbosa CG, Aparecida de Oliveira Silva J, de Jesus DV, et al. (November 2019). "Effects of Creatine Supplementation on Renal Function: A Systematic Review and Meta-Analysis". Journal of Renal Nutrition. 29 (6): 480–489. doi:10.1053/j.jrn.2019.05.004. PMID 31375416.
- Gualano B, de Salles Painelli V, Roschel H, Lugaresi R, Dorea E, Artioli GG, et al. (May 2011). "Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial". European Journal of Applied Physiology. 111 (5): 749–56. doi:10.1007/s00421-010-1676-3. PMID 20976468.
- Neves M, Gualano B, Roschel H, Lima FR, Lúcia de Sá-Pinto A, Seguro AC, et al. (June 2011). "Effect of creatine supplementation on measured glomerular filtration rate in postmenopausal women". Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquee, Nutrition et Metabolisme. 36 (3): 419–22. doi:10.1139/h11-014. PMID 21574777.
- Lugaresi R, Leme M, de Salles Painelli V, Murai IH, Roschel H, Sapienza MT, et al. (May 2013). "Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet?". Journal of the International Society of Sports Nutrition. 10 (1): 26. doi:10.1186/1550-2783-10-26. PMC 3661339. PMID 23680457.
- Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, et al. (February 2003). "Long-term creatine supplementation does not significantly affect clinical markers of health in athletes". Molecular and Cellular Biochemistry. 244 (1–2): 95–104. doi:10.1023/A:1022469320296. PMID 12701816.
- Cancela P, Ohanian C, Cuitiño E, Hackney AC (September 2008). "Creatine supplementation does not affect clinical health markers in football players". British Journal of Sports Medicine. 42 (9): 731–5. doi:10.1136/bjsm.2007.030700. PMID 18780799.
- Carvalho AP, Molina GE, Fontana KE (August 2011). "Creatine supplementation associated with resistance training does not alter renal and hepatic functions". Revista Brasileira de Medicina do Esporte. 17 (4): 237–241. doi:10.1590/S1517-86922011000400004. ISSN 1517-8692.
- Mayhew DL, Mayhew JL, Ware JS (December 2002). "Effects of long-term creatine supplementation on liver and kidney functions in American college football players". International Journal of Sport Nutrition and Exercise Metabolism. 12 (4): 453–60. doi:10.1123/ijsnem.12.4.453. PMID 12500988.
- Thorsteinsdottir B, Grande JP, Garovic VD (October 2006). "Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate". Journal of Renal Nutrition. 16 (4): 341–5. doi:10.1053/j.jrn.2006.04.025. PMID 17046619.
- Taner B, Aysim O, Abdulkadir U (February 2011). "The effects of the recommended dose of creatine monohydrate on kidney function". NDT Plus. 4 (1): 23–4. doi:10.1093/ndtplus/sfq177. PMC 4421632. PMID 25984094.
- Barisic N, Bernert G, Ipsiroglu O, Stromberger C, Müller T, Gruber S, et al. (June 2002). "Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy". Neuropediatrics. 33 (3): 157–61. doi:10.1055/s-2002-33679. PMID 12200746.
- ML.Guerrero, J.Beron, B.Spindler, P.Grosscurth, T.Wallimann and F.Verrey.Metabolic support of Na+ pump in apically permeabilized A6 kidney cell epithelia: role of creatine kinase.In: Am J Physiol. 1997 Feb;272(2 Pt 1):C697-706. doi:10.1152/ajpcell.1997.272.2.C697, PMID 9124314
- T. Wallimann, U. Riek, M. M. Möddel: Intradialytic creatine supplementation: A scientific rationale for improving the health and quality of life of dialysis patients.In: Medical Hypotheses 2017 Febr;99, S. 1-14. doi:10.1016/j.mehy.2016.12.002, PMID 28110688.
- Moreta S, Prevarin A, Tubaro F (June 2011). "Levels of creatine, organic contaminants and heavy metals in creatine dietary supplements". Food Chemistry. 126 (3): 1232–1238. doi:10.1016/j.foodchem.2010.12.028.
- Persky AM, Rawson ES (2007). Safety of creatine supplementation. Subcellular Biochemistry. 46. pp. 275–89. doi:10.1007/978-1-4020-6486-9_14. ISBN 978-1-4020-6485-2. PMID 18652082.
- "Creatine". WebMD. Natural Medicines Comprehensive Database Consumer Version. Retrieved 3 November 2014.
- Simon DK, Wu C, Tilley BC, Wills AM, Aminoff MJ, Bainbridge J, et al. (2015). "Caffeine and Progression of Parkinson Disease: A Deleterious Interaction With Creatine". Clinical Neuropharmacology. 38 (5): 163–9. doi:10.1097/WNF.0000000000000102. PMC 4573899. PMID 26366971.
- "Heterocyclic Amines in Cooked Meats". National Cancer Institute. 15 September 2004. Retrieved 9 August 2007.
- "Chemicals in Meat Cooked at High Temperatures and Cancer Risk". National Cancer Institute. 2 April 2018.
- Dahl O (1 July 1963). "Meat Quality Measurement, Creatine Content as an Index of Quality of Meat Products". Journal of Agricultural and Food Chemistry. 11 (4): 350–355. doi:10.1021/jf60128a026.
- Gießing J (20 February 2019). Kreatin: Eine natürliche Substanz und ihre Bedeutung für Muskelaufbau, Fitness und Anti-Aging. pp. 135–136, 207. ISBN 9783752803969.
External links
- Creatine bound to proteins in the PDB