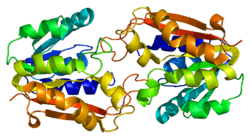
Guanidinoacetate N-methyltransferase
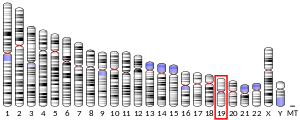
Guanidinoacetate N-methyltransferase (EC 2.1.1.2) is an enzyme that catalyzes the chemical reaction and is encoded by gene GAMT located on chromosome 19p13.3.[5]
- S-adenosyl-L-methionine + guanidinoacetate S-adenosyl-L-homocysteine + creatine
| guanidinoacetate N-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.1.1.2 | ||||||||
| CAS number | 9029-75-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Thus, the two substrates of this enzyme are S-adenosyl methionine and guanidinoacetate, whereas its two products are S-adenosylhomocysteine and creatine.
This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:N-guanidinoacetate methyltransferase. Other names in common use include GA methylpherase, guanidinoacetate methyltransferase, guanidinoacetate transmethylase, methionine-guanidinoacetic transmethylase, and guanidoacetate methyltransferase. This enzyme participates in glycine, serine and threonine metabolism and arginine and proline metabolism.
The protein encoded by this gene is a methyltransferase that converts guanidoacetate to creatine, using S-adenosylmethionine as the methyl donor. Defects in this gene have been implicated in neurologic syndromes and muscular hypotonia, probably due to creatine deficiency and accumulation of guanidinoacetate in the brain of affected individuals. Two transcript variants encoding different isoforms have been described for this gene.[5]
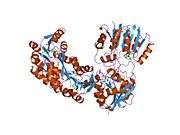
Structural studies
As of late 2007, 7 structures have been solved for this class of enzymes, with PDB accession codes 1KHH, 1P1B, 1P1C, 1XCJ, 1XCL, 1ZX0, and 2BLN.
References
- GRCh38: Ensembl release 89: ENSG00000130005 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020150 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: GAMT guanidinoacetate N-methyltransferase".
=Further reading
- Almeida LS, Vilarinho L, Darmin PS, et al. (2007). "A prevalent pathogenic GAMT mutation (c.59G>C) in Portugal". Mol. Genet. Metab. 91 (1): 1–6. doi:10.1016/j.ymgme.2007.01.005. PMID 17336114.
- Morris AA, Appleton RE, Power B, et al. (2007). "Guanidinoacetate methyltransferase deficiency masquerading as a mitochondrial encephalopathy". J. Inherit. Metab. Dis. 30 (1): 100. doi:10.1007/s10545-006-0478-2. PMID 17171576.
- Almeida LS, Rosenberg EH, Martinez-Muñoz C, et al. (2007). "Overexpression of GAMT restores GAMT activity in primary GAMT-deficient fibroblasts". Mol. Genet. Metab. 89 (4): 392–4. doi:10.1016/j.ymgme.2006.06.006. PMID 16899382.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Grimwood J, Gordon LA, Olsen A, et al. (2004). "The DNA sequence and biology of human chromosome 19". Nature. 428 (6982): 529–35. doi:10.1038/nature02399. PMID 15057824.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Komoto J, Huang Y, Hu Y, et al. (2000). "Crystallization and preliminary x-ray diffraction studies of guanidinoacetate methyltransferase from rat liver" (PDF). Acta Crystallogr. D. 55 (Pt 11): 1928–9. doi:10.1107/S0907444999010318. hdl:1808/17175. PMID 10531498.
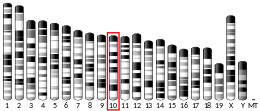
- Chae YJ, Chung CE, Kim BJ, et al. (1998). "The gene encoding guanidinoacetate methyltransferase (GAMT) maps to human chromosome 19 at band p13.3 and to mouse chromosome 10". Genomics. 49 (1): 162–4. doi:10.1006/geno.1998.5236. PMID 9570966.
- Jenne DE, Olsen AS, Zimmer M (1997). "The human guanidinoacetate methyltransferase (GAMT) gene maps to a syntenic region on 19p13.3, homologous to band C of mouse chromosome 10, but GAMT is not mutated in jittery mice". Biochem. Biophys. Res. Commun. 238 (3): 723–7. doi:10.1006/bbrc.1997.9992. PMID 9325156.
- Polyak K, Xia Y, Zweier JL, et al. (1997). "A model for p53-induced apoptosis". Nature. 389 (6648): 300–5. doi:10.1038/38525. PMID 9305847.
- Stöckler S, Isbrandt D, Hanefeld F, et al. (1996). "Guanidinoacetate methyltransferase deficiency: the first inborn error of creatine metabolism in man". Am. J. Hum. Genet. 58 (5): 914–22. PMC 1914613. PMID 8651275.
- Isbrandt D, von Figura K (1996). "Cloning and sequence analysis of human guanidinoacetate N-methyltransferase cDNA". Biochim. Biophys. Acta. 1264 (3): 265–7. doi:10.1016/0167-4781(95)00184-0. PMID 8547310.
- Cantoni GL, Scarano E (1954). "The formation of S-adenosylhomocysteine in enzymatic transmethylation reactions". J. Am. Chem. Soc. 76 (18): 4744. doi:10.1021/ja01647a081.
- CANTONI GL, VIGNOS PJ (1954). "Enzymatic mechanism of creatine synthesis". J. Biol. Chem. 209 (2): 647–59. PMID 13192118.
External links
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Guanidinoacetate N-methyltransferase