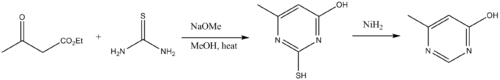Thiourea
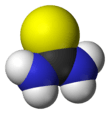
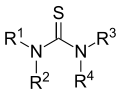
Thiourea (/ˌθaɪoʊjʊəˈriːə/)[2][3] is an organosulfur compound with the formula SC(NH2)2. It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad class of compounds with the general structure (R1R2N)(R3R4N)C=S. Thioureas are related to thioamides, e.g. RC(S)NR2, where R is methyl, ethyl, etc.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Thiourea[1] | |||
| Other names
Thiocarbamide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 605327 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.494 | ||
| 1604 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2811 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH4N2S | |||
| Molar mass | 76.12 g/mol | ||
| Appearance | white solid | ||
| Density | 1.405 g/ml | ||
| Melting point | 182 °C (360 °F; 455 K) | ||
| 142 g/l (25 °C) | |||
| −4.24×10−5 cm3/mol | |||
| Hazards | |||
EU classification (DSD) (outdated) |
Carc. Cat. 3 Repr. Cat. 3 Harmful (Xn) Dangerous for the environment (N) | ||
| R-phrases (outdated) | R22, R40, R51/53, R63 | ||
| S-phrases (outdated) | (S2), S36/37, S61 | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds |
Urea | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure and bonding
Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å.[4] The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å.
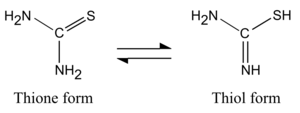
Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as Keq is 1.04×10−3.[5] The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such as isothiouronium salts.
Production
The global annual production of thiourea is around 10,000 tonnes. About 40% is produced in Germany, another 40% in China, and 20% in Japan. Thiourea can be produced from ammonium thiocyanate, but more commonly it is produced by the reaction of hydrogen sulfide with calcium cyanamide in the presence of carbon dioxide.[6]
Applications
Thiourea per se has few applications. It is mainly consumed as a precursor to thiourea dioxide, which is a common reducing agent in textile processing.[6]
Other uses
Other industrial uses of thiourea include production of flame retardant resins, and vulcanization accelerators.
Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper and almost all other types of copy paper.
It is also used to tone silver-gelatin photographic prints.
Thiourea is used in the Clifton-Phillips and Beaver bright and semi-bright electroplating processes.[7] It is also used in a solution with tin(II) chloride as an electroless tin plating solution for copper printed circuit boards.
Reactions
The material has the unusual property of changing to ammonium thiocyanate upon heating above 130 °C. Upon cooling, the ammonium salt converts back to thiourea.
Reductant
Thiourea reduces peroxides to the corresponding diols.[8] The intermediate of the reaction is an unstable endoperoxide.
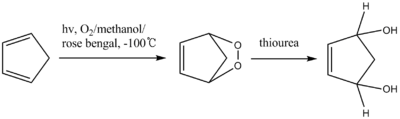
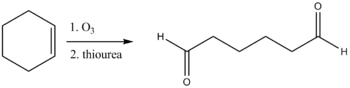
Thiourea is also used in the reductive workup of ozonolysis to give carbonyl compounds.[9] Dimethyl sulfide is also an effective reagent for this reaction, but it is highly volatile (boiling point 37 °C) and has an obnoxious odor whereas thiourea is odorless and conveniently non-volatile (reflecting its polarity).
Source of sulfide
Thiourea is employed as a source of sulfide, such as for converting alkyl halides to thiols. The reaction capitalizes on the high nucleophilicity of the sulfur center and easy hydrolysis of the intermediate isothiouronium salt:
- CS(NH2)2 + RX → RSC(NH
2)+
2X− - RSC(NH
2)+
2X−
+ 2 NaOH → RSNa + OC(NH2)2 + NaX - RSNa + HCl → RSH + NaCl
In this example, ethane-1,2-dithiol is prepared from 1,2-dibromoethane:[10]
- C2H4Br2 + 2 SC(NH2)2 → [C2H4(SC(NH2)2)2]Br2
- [C2H4(SC(NH2)2)2]Br2 + 2 KOH → C2H4(SH)2 + 2 OC(NH2)2 + 2 KBr
Like other thioamides, thiourea can serve as a source of sulfide upon reaction with metal ions. For example, mercury sulfide forms when mercuric salts in aqueous solution are treated with thiourea:
- Hg2+ + SC(NH2)2 + H2O → HgS + OC(NH2)2 + 2 H+
These sulfiding reactions, which have been applied to the synthesis of many metal sulfides, require water and typically some heating.[11][12]
Precursor to heterocycles
Thioureas are building blocks to pyrimidine derivatives. Thus thioureas condense with β-dicarbonyl compounds.[13] The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine.
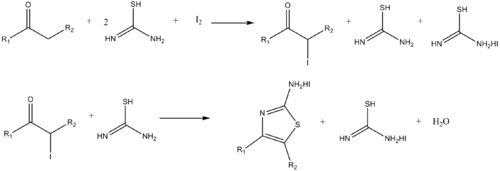
Similarly, aminothiazoles can be synthesized by the reaction of α-haloketones and thiourea.[14]
The pharmaceuticals thiobarbituric acid and sulfathiazole are prepared using thiourea.[6] 4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole is prepared by the reaction of thiourea and hydrazine.
Silver polishing
According to the label on the consumer product, the liquid silver cleaning product TarnX contains thiourea, a detergent, and sulfamic acid. A lixiviant for gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.[15]
Safety
The LD50 for thiourea is 125 mg/kg for rats (oral).[16]
A goitrogenic effect (enlargement of the thyroid gland) has been reported for chronic exposure, reflecting the ability of thiourea to interfere with iodide uptake.[6]
See also
References
- Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. 2014. pp. 98, 864. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- "Thiourea". Oxford Dictionaries UK Dictionary. Oxford University Press. Retrieved 2016-01-21.
- "Thiourea". Merriam-Webster Dictionary. Retrieved 2016-01-21.
- D. Mullen; E. Hellner (1978). "A Simple Refinement of Density Distributions of Bonding Electrons. IX. Bond Electron Density Distribution in Thiourea, CS(NH2)2, at 123K". Acta Crystallogr. B34: 2789–2794. doi:10.1107/S0567740878009243.
- Allegretti, P.E; Castro, E.A; Furlong, J.J.P (March 2000). "Tautomeric equilibrium of amides and related compounds: theoretical and spectral evidences". Journal of Molecular Structure: THEOCHEM. 499 (1–3): 121–126. doi:10.1016/S0166-1280(99)00294-8.
- Bernd Mertschenk; Ferdinand Beck; Wolfgang Bauer (2002). "Thiourea and Thiourea Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a26_803.
- 81st Universal Metal Finishing Guidebook. Metal Finishing Magazine. Fall 2013. p. 285. ISSN 0026-0576.
- C. Kaneko; A. Sugimoro & S. Tanaka (1974). "A facile one-step synthesis of cis-2-cyclopentene and cis-2-cyclohexene-1,4-diols from the corresponding cyclodienes". Synthesis. 1974 (12): 876–877. doi:10.1055/s-1974-23462.
- Gupta, D., Soman, G., and Dev, S. (1982). "Thiourea, a convenient reagent for the reductive cleavage of olefin ozonolysis products". Tetrahedron. 38 (20): 3013–3018. doi:10.1016/0040-4020(82)80187-7.CS1 maint: multiple names: authors list (link)
- Speziale, A. J. (1963). "Ethanedithiol". Organic Syntheses.; Collective Volume, 4, p. 401
- Liang, Y.; et, al. (2016). "An efficient precursor to synthesize various FeS2 nanostructures via a simple hydrothermal synthesis method". CrystEngComm. 18: 6262–6271. doi:10.1039/c6ce01203e.
- Bao, N.; et al. (2007). "Facile Cd−Thiourea Complex Thermolysis Synthesis of Phase-Controlled CdS Nanocrystals for Photocatalytic Hydrogen Production under Visible Light". The Journal of Physical Chemistry C. 111: 17527–17534. doi:10.1021/jp076566s.
- Foster, H. M., and Snyder, H. R. (1963). "4-Methyl-6-hydroxypyrimidine". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 4, p. 638
- Dodson, R. M. & King, L. C. (1945). "The reaction of ketones with halogens and thiourea". J. Am. Chem. Soc. 67 (12): 2242–2243. doi:10.1021/ja01228a059. PMID 21005695.
- Anthony Esposito. "Peñoles, UAM unveil pilot thiourea Au-Ag leaching plant - Mexico". Business News Americas (July 13, 2007).
- http://gis.dep.wv.gov/tri/cheminfo/msds1385.txt
Further reading
- Patai, S., ed. (1977). The Chemistry of double-bonded functional groups. New York, NY: John Wiley & Sons. pp. 1355–1496. ISBN 0-471-92493-8.