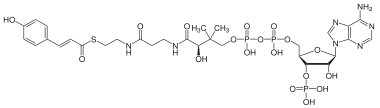
Coumaroyl-CoA
Coumaroyl-coenzyme A is a chemical compound found in plants. The compound is the thioester of coenzyme-A and coumaric acid.
 | |
| Names | |
|---|---|
| IUPAC name
S-[2-[3-[[4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,
3-dimethylbutanoyl]amino]propanoylamino]ethyl](E)-3-(4-hydroxyphenyl)prop-2-enethioate | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C30H42N7O18P3S | |
| Molar mass | 913.67 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis and significance
It is generated in nature from phenylalanine, which is converted by PAL to trans-cinnamate. Trans-cinnamate is hydroxylated by trans-cinnamate 4-monooxygenase to give 4-hydroxycinnamate (i.e, coumarate). Coumarate is condensed with coenzyme-A in the presence of 4-coumarate-CoA ligase:
- ATP + 4-coumarate + CoA AMP + diphosphate + 4-coumaroyl-CoA.
Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and other phenylpropanoids.[1]
Enzymes using Coumaroyl-Coenzyme A
- Anthocyanin 3-O-glucoside 6''-O-hydroxycinnamoyltransferase
- Anthocyanin 5-aromatic acyltransferase
- Chalcone synthase
- 4-Coumarate-CoA ligase
- 6'-Deoxychalcone synthase
- Agmatine N4-coumaroyltransferase
- Flavonol-3-O-triglucoside O-coumaroyltransferase
- Naringenin-chalcone synthase
- Shikimate O-hydroxycinnamoyltransferase
- Trihydroxystilbene synthase
gollark: The time of giannises was very confusing.
gollark: I figure YouTube is bound to fail eventually since it's trying to handle too many conflicting demands from various sides and not handling any that well. But for now it sort of works.
gollark: You could probably use non-youtube video hosting, but it would be annoying and hard to monetize.
gollark: I would assume making the images out of them takes some work, unless there's a convenient script for that.
gollark: Do you just spend ages manually compiling these big collages of comemnts?
References
- Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3: 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.