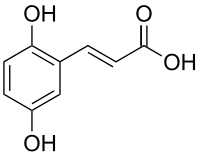
2,5-Dihydroxycinnamic acid
2,5-Dihydroxycinnamic acid is a hydroxycinnamic acid. It is an isomer of caffeic acid.
 | |
| Names | |
|---|---|
| IUPAC name
3-(2,5-dihydroxyphenyl)prop-2-enoic acid | |
| Other names
(2E)-3-(2,5-Dihydroxyphenyl)acrylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C9H8O4 | |
| Molar mass | 180.159 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
2,5-Dihydroxycinnamic acid is produced by Elbs persulfate oxidation of o-Coumaric acid.[1][2]
gollark: There's a mod for it, EnderNet or something.
gollark: It would be neat if you could transfer items across servers though. Obviously very problematic, but neat.
gollark: I don't think having server admins setting prices is a good idea though.
gollark: I suppose it being cross-server could be interesting.
gollark: I mean, are there not decent economy plugins for servers already?
See also
References
- Cain, J.C.; Greenaway, A.J. (1907). "Abstracts of Papers on Organic chemistry". Journal of the Chemical Society, Abstracts. 92: A741–A812. doi:10.1039/CA9079200741.
- Otto, Neubauer; Flatow, L. (1907). "Synthesen von Alkaptonsäuren". Zeitschrift für Physiologische Chemie. 52 (3–4): 375–398. doi:10.1515/bchm2.1907.52.3-4.375.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.