Dihydroquinidine
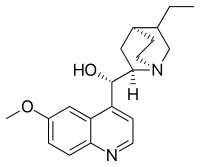
Dihydroquinidine (also called hydroquinidine) is an organic compound, a cinchona alkaloid closely related to quinine. The specific rotation is +226° in ethanol at 2g/100 ml. A derivative of this molecule is used as chiral ligand in the AD-mix for Sharpless dihydroxylation.
 | |
| Names | |
|---|---|
| IUPAC name
(S)-[(2R,4S,5R)-5-ethyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol | |
| Other names
(9S)-10,11-Dihydro-6'-methoxycinchonan-9-ol | |
| Identifiers | |
| ChEMBL | |
| ECHA InfoCard | 100.007.578 |
PubChem CID |
|
| UNII | |
| Properties | |
| C20H26N2O2 | |
| Molar mass | 326.4 g/mol |
| Melting point | 169 to 172 °C (336 to 342 °F; 442 to 445 K) |
| Pharmacology | |
| C01BA13 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The substance is also a class Ia antiarrhythmic medication.[1]
See also
References
- Drugs.com: International Drug Names for hydroquinidine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.