History of fertilizer
The history of fertilizer has largely shaped political, economic, and social circumstances in their traditional uses. Subsequently, there has been a radical reshaping of environmental conditions following the development of chemically synthesized fertilizers.[1][2][3]
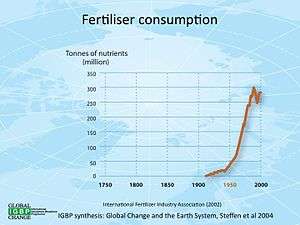
History
Egyptians, Romans, Babylonians, and early Germans all are recorded as using minerals and/or manure to enhance the productivity of their farms. The use of wood ash as a field treatment became widespread.[4]
In the 19th century, guano, which had been known and used in the Andes for at least 1500 years, was taken in large quantities from Peru and Chile (and later also from Namibia and other areas) to Europe and the USA.
Key figures in Europe
In the 1730s, Viscount Charles Townshend (1674–1738) first studied the improving effects of the four crop rotation system that he had observed in use in Flanders. For this he gained the nickname of Turnip Townshend.
Johann Fredrich Mayer
Johann Friedrich Mayer (1719–1798) was the first to present to the world a series of experiments upon it the relation of gypsum to agriculture, and many chemists have followed him in the 19th century. Early 19th century however a great variety of opinion remained with regard to its mode of operation, for example:[5]
- The French agronomist Victor Yvart (1763–1831)[6] believed that the action of gypsum is exclusively the effect of the sulphuric acid, which enters into its composition; and founds this opinion upon the fact that the ashes of turf, which contain sulphate of iron and sulphate of alumina, have the same action upon vegetation as gypsum.[5]
- The French agronomist Charles Philibert de Lasteyrie (1759–1849), observing that plants whose roots were nearest the surface of the soil were most acted upon by plaster, concludes that gypsum takes from the atmosphere the elements of vegetable life, and transmits them directly to plants.[5]
- Louis Augustin Guillaume Bosc intimates that the septic quality of gypsum (which he takes for granted) best explains its action on vegetation; but this opinion is subverted by the experiments of Davy.[5]
- Humphry Davy found that, of two parcels of minced veal, the one mixed with gypsum, the other left by itself, and both exposed to the action of the sun, the latter was the first to exhibit symptoms of putrefaction. Davy's own belief on this subject is, that it makes part of the food of vegetables, is received into the plant, and combined with it.[5]
Mayer also promote new regimes of crop rotation.[7]
Justus von Liebig
Chemist Justus von Liebig (1803–1873) contributed greatly to the advancement in the understanding of plant nutrition. His influential works first denounced the vitalist theory of humus, arguing first the importance of ammonia, and later promoting the importance of inorganic minerals to plant nutrition.[8] Primarily Liebig's work succeeded in exposition of questions for agricultural science to address over the next 50 years.
In England, he attempted to implement his theories commercially through a fertilizer created by treating phosphate of lime in bone meal with sulfuric acid. Although it was much less expensive than the guano that was used at the time, it failed because it was not able to be properly absorbed by crops.
Sir John Bennet Lawes
John Bennet Lawes, an English entrepreneur, (view timeline of his life and work) began to experiment on the effects of various manures on plants growing in pots in 1837, and a year or two later the experiments were extended to crops in the field. One immediate consequence was that in 1842 he patented a manure formed by treating phosphates with sulphuric acid, and thus was the first to create the artificial manure industry.[9] In the succeeding year he enlisted the services of Joseph Henry Gilbert, who had studied under Liebig at the University of Giessen, as director of research at the Rothamsted Experimental Station which he founded on his estate. To this day, the Rothamsted research station the pair founded still investigates the impact of inorganic and organic fertilizers on crop yields.[10]
Jean Baptiste Boussingault
In France, Jean Baptiste Boussingault (1802–1887) pointed out that the amount of nitrogen in various kinds of fertilizers is important.
Metallurgists Percy Gilchrist (1851–1935) and Sidney Gilchrist Thomas (1850–1885) invented the Gilchrist-Thomas process, which enabled the use of high phosphorus acidic Continental ores for steelmaking. The dolomite lime lining of the converter turned in time into calcium phosphate, which could be used as fertilizer, known as Thomas-phosphate.
The Birkeland-Eyde Process
The Birkeland–Eyde process was developed by Norwegian industrialist and scientist Kristian Birkeland along with his business partner Sam Eyde in 1903, based on a method used by Henry Cavendish in 1784.[11] This process was used to fix atmospheric nitrogen (N2) into nitric acid (HNO3), one of several chemical processes generally referred to as nitrogen fixation. The resultant nitric acid was then used for the production of synthetic fertilizer. A factory based on the process was built in Rjukan and Notodden in Norway, combined with the building of large hydroelectric power facilities.[12] The process is inefficient in terms of energy usage, and is today replaced by the Haber process.[13]
The Haber Process
In the early decades of the 20th century, the Nobel prize-winning chemists Carl Bosch of IG Farben and Fritz Haber developed the Haber process[14] which utilized molecular nitrogen (N2) and methane (CH4) gas in an economically sustainable synthesis of ammonia (NH3). The ammonia produced in the Haber process is the main raw material of the Ostwald process.
The Ostwald process
The Ostwald process is a chemical process for production of nitric acid (HNO3), which was developed by Wilhelm Ostwald (patented 1902). It is a mainstay of the modern chemical industry and provides the raw material for the most common type of fertilizer production, globally (for example, ammonium nitrate, a common fertilizer, is made by reacting ammonia with nitric acid). Historically and practically it is closely associated with the Haber process, which provides the requisite raw material, ammonia (NH3).
Erling Johnson
In 1927 Erling Johnson developed an industrial method for producing nitrophosphate, also known as the Odda process after his Odda Smelteverk of Norway. The process involved acidifying phosphate rock (from Nauru and Banaba Islands in the southern Pacific Ocean) with nitric acid to produce phosphoric acid and calcium nitrate which, once neutralized, could be used as a nitrogen fertilizer.
Industry
British
The developing sciences of chemistry and Paleontology, combined with the discovery of coprolites in commercial quantities in East Anglia, led Fisons and Packard to develop sulfuric acid and fertilizer plants at Bramford, and Snape, Suffolk in the 1850s to create superphosphates, which were shipped around the world from the port at Ipswich. By 1871 there were about 80 factories making superphosphate.[15]
After World War I these businesses came under competitive pressure from naturally produced guano, primarily found on the Pacific islands, as their extraction and distribution had become economically attractive.
The interwar period[16] saw innovative competition from Imperial Chemical Industries who developed synthetic ammonium sulfate in 1923, Nitro-chalk in 1927, and a more concentrated and economical fertilizer called CCF (Concentrated Complete Fertiliser) based on ammonium phosphate in 1931.[17] Competition was limited as ICI ensured it controlled most of the world's ammonium sulfate supplies.
North America and other European Countries
Other European and North American fertilizer companies developed their market share, forcing the English pioneer companies to merge, becoming Fisons, Packard, and Prentice Ltd. in 1929. Together they produced 85,000 tons of superphosphate/year in 1934 from their new factory and deep-water docks in Ipswich. By World War II they had acquired about 40 companies, including Hadfields in 1935, and two years later the large Anglo-Continental Guano Works, founded in 1917.
The post-war environment was characterized by much higher production levels as a result of the "Green Revolution" and new types of seed with increased nitrogen-absorbing potential, notably the high-response varieties of maize, wheat, and rice. This has accompanied the development of strong national competition, accusations of cartels and supply monopolies, and ultimately another wave of mergers and acquisitions. The original names no longer exist other than as holding companies or brand names: Fisons and ICI agrochemicals are part of today's Yara International[18] and AstraZeneca companies.
Major players in this market now include the Russian fertilizer company Uralkali (listed on the London Stock Exchange), whose former majority owner is Dmitry Rybolovlev, ranked by Forbes as 60th in the list of wealthiest people in 2008.
See also
References
- Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. MIT Press. ISBN 9780262693134.
- Curtis, Harry A. (1924). "Fertilizers: The World Supply". Foreign Affairs. 2 (3): 436–445. doi:10.2307/20028312. JSTOR 20028312.
- Brand, Charles J. (1945). "Some Fertilizer History Connected with World War I". Agricultural History. 19 (2): 104–113. JSTOR 3739556.
- Heinrich W. Scherer "Fertilizers" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_323.pub3
- John Armstrong, Jesse Buel. A Treatise on Agriculture, The Present Condition of the Art Abroad and at Home, and the Theory and Practice of Husbandry. To which is Added, a Dissertation on the Kitchen and Garden. 1840. p. 45.
- SeeVictor Yvart at the French Wikipedia
- Günter Rudolf Golde (1975) Catholics and Protestants: agricultural modernization in two German villages. p. 15
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 16 (11th ed.). Cambridge University Press.
-

- "Classical Experiments". Rothamsted Research. Retrieved 1 September 2014.
- Aaron John Ihde (1984). The development of modern chemistry. Courier Dover Publications. p. 678. ISBN 0486642356.
- G. J. Leigh (2004). The world's greatest fix: a history of nitrogen and agriculture. Oxford University Press US. pp. 134–139. ISBN 0195165829.
- Trevor Illtyd Williams; Thomas Kingston Derry (1982). A short history of twentieth-century technology c. 1900-c. 1950. Oxford University Press. pp. 134–135. ISBN 0198581599.
- Haber & Bosch Most influential persons of the 20th century, by Jürgen Schmidhuber
- "Fisons at the root of modern agriculture - Yara". 20 May 2006. Archived from the original on 20 May 2006.
- "Competition Commission report" (PDF). Archived from the original (PDF) on 27 March 2009. Retrieved 18 November 2009.
- "Fertilisers". GANSG - Agricultural Merchants and Fertiliser Industries. Retrieved 1 October 2016.
- "History of Yara at Yara.com". Archived from the original on 28 September 2007. Retrieved 18 November 2009.