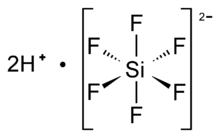
Hexafluorosilicic acid
Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6 also written as (H
3O)
2[SiF
6]. It is a colorless liquid mostly encountered as diluted aqueous solution, from there, the second chemical notation also proposed. Hexafluorosilicic acid has a distinctive sour taste and pungent smell. It is produced naturally on a large scale in volcanoes.[1][2] It is manufactured as a coproduct in the production of phosphate fertilizers. The resulting hexafluorosilicic acid is almost exclusively consumed as a precursor to aluminum trifluoride and synthetic cryolite, which are used in aluminium processing. Salts derived from hexafluorosilicic acid are called hexafluorosilicates.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexafluorosilicic acid | |
| Systematic IUPAC name
Dihydrogen hexafluorosilicate | |
| Other names
Fluorosilicic acid, fluosilic acid, hydrofluorosilicic acid, silicofluoride, silicofluoric acid, oxonium hexafluorosilanediuide, oxonium hexafluoridosilicate(2−) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.037.289 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1778 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| F6H2Si | |
| Molar mass | 144.091 g·mol−1 |
| Appearance | transparent, colorless, fuming liquid |
| Odor | sour, pungent |
| Density | 1.22 g/cm3 (25% soln.) 1.38 g/cm3 (35% soln.) 1.46 g/cm3 (61% soln.) |
| Melting point | ca. 19 °C (66 °F; 292 K) (60–70% solution) < −30 °C (−22 °F; 243 K) (35% solution) |
| Boiling point | 108.5 °C (227.3 °F; 381.6 K) (decomposes) |
| miscible | |
Refractive index (nD) |
1.3465 |
| Structure | |
| Octahedral SiF62− | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| GHS Signal word | Danger |
GHS hazard statements |
H314 |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
430 mg/kg (oral, rat) |
| Related compounds | |
Other cations |
Ammonium hexafluorosilicate |
Related compounds |
Hexafluorophosphoric acid Fluoroboric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
Hexafluorosilicic acid is generally assumed to consist of oxonium ions charge balanced by hexafluorosilicate dianions as well as water. In aqueous solution, the hydronium cation (H3O+) is traditionally equated with a solvated proton, and as such, the formula for this compound is often written as H
2SiF
6. Extending that metaphor, the isolated compound is then written as H
2SiF
6·2H
2O, or (H
3O)
2SiF
6.The situation is similar to that for chloroplatinic acid, fluoroboric acid, and hexafluorophosphoric acid. Hexafluorosilicate is an octahedral anion; the Si–F bond distances are 1.71 Å.[3] Hexafluorosilicic acid is only available commercially as solution.[4]
Production and principal reactions
The commodity chemical hydrogen fluoride is produced from fluorite by treatment with sulfuric acid.[5] As a by-product, approximately 50 kg of (H3O)2SiF6 is produced per tonne of HF owing to reactions involving silica-containing mineral impurities. (H3O)2SiF6 is also produced as a by-product from the production of phosphoric acid from apatite and fluorapatite. Again, some of the HF in turn reacts with silicate minerals, which are an unavoidable constituent of the mineral feedstock, to give silicon tetrafluoride. Thus formed, the silicon tetrafluoride reacts further with HF. The net process can be described as:[6]
- SiO
2 + 6 HF → SiF2−
6 + 2 H
3O+
Hexafluorosilicic acid can also be produced by treating silicon tetrafluoride with hydrofluoric acid.
In water, hexafluorosilicic acid readily hydrolyzes to hydrofluoric acid and various forms of amorphous and hydrated silica ("SiO2"). At the concentration usually used for water fluoridation, 99% hydrolysis occurs and the pH drops. The rate of hydrolysis increases with pH. At the pH of drinking water, the degree of hydrolysis is essentially 100%.[7]
- H2SiF6 + 2 H2O → 6 HF + "SiO2"
Neutralization of solutions of hexafluorosilicic acid with alkali metal bases produces the corresponding alkali metal fluorosilicate salts:
- (H3O)2SiF6 + 2 NaOH → Na2SiF6 + 4 H2O
The resulting salt Na2SiF6 is mainly used in water fluoridation. Related ammonium and barium salts are produced similarly for other applications.
Near neutral pH, hexafluorosilicate salts hydrolyze rapidly according to this equation:[8]
- SiF2−
6 + 2 H2O → 6 F− + SiO2 + 4 H+
Uses
The majority of the hexafluorosilicic acid is converted to aluminium fluoride and cryolite.[6] These materials are central to the conversion of aluminium ore into aluminium metal. The conversion to aluminium trifluoride is described as:
- H2SiF6 + Al2O3 → 2 AlF3 + SiO2 + H2O
Hexafluorosilicic acid is also converted to a variety of useful hexafluorosilicate salts. The potassium salt, Potassium fluorosilicate, is used in the production of porcelains, the magnesium salt for hardened concretes and as an insecticide, and the barium salts for phosphors.
Hexafluorosilicic acid is also used as an electrolyte in the Betts electrolytic process for refining lead.
Hexafluorosilicic acid (identified as hydrofluorosilicic acid on the label) along with oxalic acid are the active ingredients used in Iron Out rust-removing cleaning products, which are essentially varieties of laundry sour.
Niche applications
H2SiF6 is a specialized reagent in organic synthesis for cleaving Si–O bonds of silyl ethers. It is more reactive for this purpose than HF. It reacts faster with t-butyldimethysilyl (TBDMS) ethers than triisopropylsilyl (TIPS) ethers.[9]
Hexafluorosilicic acid and the salts are used as wood preservation agents.[10]
Natural salts
Some rare minerals, encountered either within volcanic or coal-fire fumaroles, are salts of the hexafluorosilicic acid. Examples include ammonium hexafluorosilicate that naturally occurs as two polymorphs: cryptohalite and bararite.[11][12][13]
Safety
Hexafluorosilicic acid can release hydrogen fluoride when evaporated, so it has similar risks. Inhalation of the vapors may cause lung edema. Like hydrogen fluoride, it attacks glass and stoneware.[14] The LD50 value of hexafluorosilicic acid is 430 mg/kg.[15]
References
- Palache, C., Berman, H., and Frondel, C. (1951) Dana’s System of Mineralogy, Volume II: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, etc. John Wiley and Sons, Inc., New York, 7th edition.
- Anthony, J.W., Bideaux, R.A., Bladh, K.W., and Nichols, M.C. (1997) Handbook of Mineralogy, Volume III: Halides, Hydroxides, Oxides. Mineral Data Publishing, Tucson.
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- J. P. Nicholson (2005). "Electrodeposition of Silicon from Nonaqueous Solvents". J. Electrochem. Soc. 152 (12): C795–C802. doi:10.1149/1.2083227.
- USGS. Fluorspar.
- Aigueperse, J.; Mollard, P.; Devilliers, D.; Chemla, M.; Faron, R.; Romano, R.; Cuer, J. P. "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307.
- "Sodium Hexafluorosilicate [CASRN 16893-85-9] and Fluorosilicic Acid [CASRN 16961-83-4] Review of Toxicological Literature" (PDF). ntp.niehs.nih.gov. Retrieved July 13, 2017.
- Finney, William F.; Wilson, Erin; Callender, Andrew; Morris, Michael D.; Beck, Larry W. (2006). "Reexamination of Hexafluorosilicate Hydrolysis by 19F NMR and pH Measurement". Environ. Sci. Technol. 40 (8): 2572–2577. Bibcode:2006EnST...40.2572F. doi:10.1021/es052295s.
- Pilcher, A. S.; DeShong, P. (2001). "Fluorosilicic Acid". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rf013. ISBN 0471936235.
- Carsten Mai, Holger Militz (2004). "Modification of wood with silicon compounds. inorganic silicon compounds and sol-gel systems: a review". Wood Science and Technology. 37 (5): 339. doi:10.1007/s00226-003-0205-5.
- https://www.mindat.org/min-1163.html
- https://www.mindat.org/min-511.html
- Kruszewski, Ł., Fabiańska, M.J., Segit, T., Kusy, D., Motyliński, R., Ciesielczuk, J., Deput, E., 2020. Carbon-nitrogen compounds, alcohols, mercaptans, monoterpenes, acetates, aldehydes, ketones, SF6, PH3, and other fire gases in coal-mining waste heaps of Upper Silesian Coal Basin (Poland) – a re-investigation by means of in-situ FTIR extrernal database approach. Sci. Total Env., 698, 134274, doi: 10.1016/j.scitotenv.2019.134274
- "CDC – Fluorosilicic Acid – International Chemical Safety Cards - NIOSH". Cdc.gov. Retrieved 2015-03-10.
- Archived October 22, 2012, at the Wayback Machine
