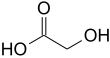
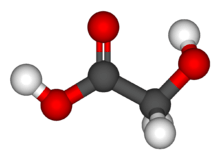
Glycolic acid
Glycolic acid (hydroacetic acid or hydroxyacetic acid); chemical formula C2H4O3 (also written as HOCH2CO2H), is the smallest α-hydroxy acid (AHA). This colorless, odorless, and hygroscopic crystalline solid is highly soluble in water. It is used in various skin-care products. Glycolic acid is found in some sugar-crops.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Hydroxyethanoic acid | |||
| Preferred IUPAC name
hydroxyacetic acid | |||
| Other names
dicarbonous acid glycolic acid hydroacetic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.073 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4O3 | |||
| Molar mass | 76.05 g/mol | ||
| Appearance | white, powdery solid | ||
| Density | 1.49 g/cm3[1] | ||
| Melting point | 75 °C (167 °F; 348 K) | ||
| Boiling point | decomposes | ||
| 70% solution | |||
| Solubility in other solvents | alcohols, acetone, acetic acid and ethyl acetate[2] | ||
| log P | -1.05[3] | ||
| Acidity (pKa) | 3.83 | ||
| Hazards | |||
| Main hazards | Corrosive (C) | ||
| R-phrases (outdated) | R22-R34 | ||
| S-phrases (outdated) | S26-S36/37/39-S45 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 129 °C (264 °F; 402 K) [4] | ||
| Related compounds | |||
Related α-hydroxy acids |
Lactic acid | ||
Related compounds |
Glycolaldehyde Acetic acid Glycerol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
A glycolate or glycollate is a salt or ester of glycolic acid.
History
The name "glycolic acid" was coined in 1848 by French chemist Auguste Laurent (1807–1853). He proposed that the amino acid glycine—which was then called glycocolle—might be the amine of a hypothetical acid, which he called "glycolic acid" (acide glycolique).[5]
Glycolic acid was first prepared in 1851 by German chemist Adolph Strecker (1822–1871) and Russian chemist Nikolai Nikolaevich Sokolov (1826–1877). They produced it by treating hippuric acid with nitric acid and nitrogen dioxide to form an ester of benzoic acid and glycolic acid (C6H5C(=O)OCH2COOH), which they called "benzoglycolic acid" (Benzoglykolsäure; also benzoyl glycolic acid). They boiled the ester for days with dilute sulfuric acid, thereby obtaining benzoic acid and glycolic acid (Glykolsäure).[6][7]
Preparation
Glycolic acid can be synthesized in various ways. The predominant approaches use a catalyzed reaction of formaldehyde with synthesis gas (carbonylation of formaldehyde), for its low cost.[8]
It is also prepared by the reaction of chloroacetic acid with sodium hydroxide followed by re-acidification.
Other methods, not noticeably in use, include hydrogenation of oxalic acid, and hydrolysis of the cyanohydrin derived from formaldehyde.[9] Some of today's glycolic acids are formic acid-free. Glycolic acid can be isolated from natural sources, such as sugarcane, sugar beets, pineapple, cantaloupe and unripe grapes.[10]
Glycolic acid can also be prepared using an enzymatic biochemical process that may require less energy.[11]
Properties
Glycolic acid is slightly stronger than acetic acid due to the electron-withdrawing power of the terminal hydroxyl group. The carboxylate group can coordinate to metal ions forming coordination complexes. Of particular note are the complexes with Pb2+ and Cu2+ which are significantly stronger than complexes with other carboxylic acids. This indicates that the hydroxyl group is involved in complex formation, possibly with the loss of its proton.[12]
Applications
Glycolic acid is used in the textile industry as a dyeing and tanning agent,[13] in food processing as a flavoring agent and as a preservative, and in the pharmaceutical industry as a skin care agent. It is also used in adhesives and plastics.[14] Glycolic acid is often included in emulsion polymers, solvents and additives for ink and paint in order to improve flow properties and impart gloss. It is used in surface treatment products that increase the coefficient of friction on tile flooring. It is the active ingredient in the household cleaning liquid Pine-Sol.
Skin care
Due to its capability to penetrate skin, glycolic acid finds applications in skin care products, most often as a chemical peel. Physician-strength peels can have a pH as low as 0.6 (strong enough to completely keratolyze the epidermis), while acidities for home peels can be as low as 2.5. Once applied, glycolic acid reacts with the upper layer of the epidermis, weakening the binding properties of the lipids that hold the dead skin cells together. This allows the stratum corneum to be exfoliated, exposing live skin cells.
Organic synthesis
Glycolic acid is a useful intermediate for organic synthesis, in a range of reactions including: oxidation-reduction, esterification and long chain polymerization. It is used as a monomer in the preparation of polyglycolic acid and other biocompatible copolymers (e.g. PLGA). Commercially, important derivatives include the methyl (CAS# 96-35-5) and ethyl (CAS# 623-50-7) esters which are readily distillable (boiling points 147–149 °C and 158–159 °C, respectively), unlike the parent acid. The butyl ester (b.p. 178–186 °C) is a component of some varnishes, being desirable because it is nonvolatile and has good dissolving properties.[9]
Agriculture
Many plants make glycolic acid during photorespiration. Its role consumes significant amounts of energy. In 2017 researchers announced a process that employs a novel protein to reduce energy consumption/loss and prevent plants from releasing harmful ammonia. The process converts glycolate into glycerate without using the conventional BASS6 and PLGG1 route.[15][16]
Safety
Glycolic acid is a strong irritant depending on pH.[17] Like ethylene glycol, it is metabolized to oxalic acid, which could make it dangerous if ingested.
References
- United States National Library of Medicine "Hydroxyacetic Acid" in TOXNET Hazardous Substances Data Bank (HSDB), citing Gerhartz, W. (exec ed.), Ullmann's Encyclopedia of Industrial Chemistry. 5th ed.Vol A1: Deerfield Beach, FL: VCH Publishers, 1985 to Present., p. VA13 509.
- "DuPont Glycolic Acid Technical Information". Archived from the original on 2006-07-14. Retrieved 2006-07-06.
- "Glycolic acid_msds".
- "Glycolic Acid MSDS". University of Akron. Retrieved 2006-09-18.
- Laurent, Auguste (1848). "Sur les acides amidés et le sucre de gélatine" ("On aminated acids and the sugar of gelatine [i.e., glycine]"), Annales de Chimie et de Physique, 3rd series, 23: 110–123. From p. 112: "Appelons ce dernier acide glycolique ... " ("Let us call the latter 'glycolic acid' ...")
- Socoloff, Nicolaus and Strecker, Adolph (1851) "Untersuchung einiger aus der Hippursäure entstehenden Producte" ("Investigation of some products that arise from hippuric acid"), Annalen der Chemie und Pharmacie, 80: 17–43. For their production of glycolic acid, see pp. 34–37. Note: Strecker and Sokolov's empirical formula for glycolic acid (viz, C4H4O6) was incorrect, because like many chemists at that time, they used the wrong atomic masses for carbon (6 instead of 12) and for oxygen (8 instead of 16).
- (Socoloff and Strecker, 1851), p. 37. In recognition of Laurent's correct surmise, Strecker and Sokolov named glycolic acid: "Die in dem Barytsalz enthaltene Säure C4H3O5 oder als Säurehydrat gedacht C4H4O6 kommt mit der Säure überein, als deren Amidverbindung man das Glycocoll betrachten kann, und welche daher von Laurent den Namen Glycolsäure erhalten hat." (The acid C4H3O5 contained in the barium salt — or considered as the acid hydrate C4H4O6 — is consistent with the acid whose amide can be regarded as glycocoll and which therefore obtained from Laurent the name "glycolic acid".)
- D.J. Loder, U.S. Patent 2,152,852 (1939).
- Karlheinz Miltenberger "Hydroxycarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- "Glycolic acid, What is Glycolic acid? About its Science, Chemistry and Structure". www.3dchem.com. Retrieved 2018-04-11.
- Thaipolychemicals
- Sigel, Astrid; Operschall, Bert P.; Sigel, Helmut (2017). "Chapter 11. Complex Formation of Lead(II) with Nucleotides and Their Constituents". In Astrid, S.; Helmut, S.; Sigel, R. K. O. (eds.). Lead: Its Effects on Environment and Health. Metal Ions in Life Sciences. 17. de Gruyter. pp. 319–402. doi:10.1515/9783110434330-011.
- http://www2.dupont.com/Glycolic_Acid/en_US/uses_apps/industrial/ind_pgs/leather_tanning.html
- thefreedictionary.com
- GEREA, ALEXANDRA (2017-04-03). "New protein can increase yields, save farmers millions every year". ZME Science. Retrieved 2017-04-06.
- South, Paul F.; Walker, Berkley J.; Cavanagh, Amanda P.; Rolland, Vivien; Badger, Murray; Ort, Donald R. (2017-03-28). "Bile Acid Sodium Symporter BASS6 Can Transport Glycolate and Is Involved in Photorespiratory Metabolism in Arabidopsis thaliana". The Plant Cell. 29: tpc.00775.2016. doi:10.1105/tpc.16.00775. ISSN 1532-298X. PMC 5435425. PMID 28351992.
- "Glycolic Acid MSDS". ICSC:NENG1537 International Chemical Safety Cards (WHO/IPCS/ILO). CDC/NIOSH. Retrieved 2006-06-08.