Geomicrobiology
Geomicrobiology is the scientific field at the intersection of geology and microbiology. It concerns the role of microbes on geological and geochemical processes and effects of minerals and metals to microbial growth, activity and survival.[2] Such interactions occur in the geosphere (rocks, minerals, soils, and sediments), the atmosphere and the hydrosphere.[3] Geomicrobiology studies microorganisms that are driving the Earth's biogeochemical cycles, mediating mineral precipitation and dissolution, and sorbing and concentrating metals. [4] The applications include for example bioremediation[5], mining, climate change mitigation[6] and public drinking water supplies.[7]
Rocks and minerals
Microbe-aquifer interactions
Microorganisms are known to impact aquifers by modifying their rates of dissolution. In the karstic Edwards Aquifer, microbes colonizing the aquifer surfaces enhance the dissolution rates of the host rock.[8]
In the oceanic crustal aquifer, the largest aquifer on Earth,[9] microbial communities can impact ocean productivity, sea water chemistry as well as geochemical cycling throughout the geosphere. The mineral make-up of the rocks affects the composition and abundance of these subseafloor microbial communities present.[10] Through bioremediation some microbes can aid in decontaminating freshwater resources in aquifers contaminated by waste products.
Microbially precipitated minerals
Some bacteria use metal ions as their energy source. They convert (or chemically reduce) the dissolved metal ions from one electrical state to another. This reduction releases energy for the bacteria's use, and, as a side product, serves to concentrate the metals into what ultimately become ore deposits. Biohydrometallurgy or in situ mining is where low-grade ores may be attacked by well-studied microbial processes under controlled conditions to extract metals. Certain iron, copper, uranium and even gold ores are thought to have formed as the result of microbe action.[11]
Subsurface environments, like aquifers, are attractive locations when selecting repositories for nuclear waste, carbon dioxide (See carbon sequestration), or as artificial reservoirs for natural gas. Understanding microbial activity within the aquifer is important since it may interact with and effect the stability of the materials within the underground repository.[12] Microbe-mineral interactions contribute to biofouling and microbially induced corrosion. Microbially induced corrosion of materials, such as carbon steel, have serious implications in the safe storage of radioactive waste within repositories and storage containers.[13]
Environmental remediation
Microbes are being studied and used to degrade organic and even nuclear waste pollution (see Deinococcus radiodurans) and assist in environmental cleanup. An application of geomicrobiology is bioleaching, the use of microbes to extract metals from mine waste.
Soil and sediment: microbial remediation

Microbial remediation is used in soils to remove contaminants and pollutants. Microbes play a key role in many biogeochemistry cycles and can effect a variety of soil properties, such as biotransformation of mineral and metal speciation, toxicity, mobility, mineral precipitation, and mineral dissolution. Microbes play a role in the immobilization and detoxification of a variety of elements, such as metals, radionuclides, sulfur and phosphorus, in the soil.Thirteen metals are considered priority pollutants (Sb, As, Be, Cd, Cr, Cu, Pb, Ni, Se, Ag, Tl, Zn, Hg).[2] Soils and sediment act as sinks for metals which originate from both natural sources through rocks and minerals as well as anthropogenic sources through agriculture, industry, mining, waste disposal, among others.
Many heavy metals, such as chromium (Cr), at low concentrations are essential micronutrients in the soil, however they can be toxic at higher concentrations. Heavy metals are added into soils through many anthropogenic sources such industry and/or fertilizers. Heavy metal interaction with microbes can increase or decrease the toxicity. Levels of chromium toxicity, mobility and bioavailability depend on oxidation states of chromium.[14] Two of the most common chromium species are Cr(III) and Cr(VI). Cr(VI) is highly mobile, bioavailable and more toxic to flora and fauna, while Cr(III) is less toxic, more immobile and readily precipitates in soils with pH >6.[15] Utilizing microbes to facilitate the transformation of Cr(VI) to Cr(III) is an environmentally friendly, low cost bioremediation technique to help mitigate toxicity in the environment.[16]
Acid mine drainage
Another application of geomicrobiology is bioleaching, the use of microbes to extract metals from mine waste. For example, sulfate-reducing bacteria (SRB) produce H2S which precipitates metals as a metal sulfide. This process removed heavy metals from mine waste which is one of the major environmental issues associated with acid mine drainage (along with a low pH).[17]
Bioremediation techniques are also used on contaminated surface water and ground water often associated with acid mine drainage. Studies have shown that the production of bicarbonate by microbes such as sulfate-reducing bacteria adds alkalinity to neutralize the acidity of the mine drainage waters.[5] Hydrogen ions are consumed while bicarbonate is produced which leads to an increase in pH (decrease in acidity).[18]
Microbial degradation of hydrocarbons
Microbes can affect the quality of oil and gas deposits through their metabolic processes.[19] Microbes can influence the development of hydrocarbons by being present at the time of deposition of the source sediments or by dispersing through the rock column to colonize reservoir or source lithologies after the generation of hydrocarbons.
Early Earth history and astrobiology
_1_(17346619166).jpg)
A common field of study within geomicrobiology is origin of life on earth or other planets. Various rock-water interactions, such as serpentinization and water radiolysis,[12] are possible sources of metabolic energy to support chemolithoautotrophic microbial communities on Early Earth and on other planetary bodies such as Mars, Europa and Enceladus.[20][21]
Interactions between microbes and sediment record some of the earliest evidence of life on earth. Information on the life during Archean Earth is recorded in bacterial fossils and stromatolites preserved in precipitated lithologies such as chert or carbonates.[22][23] Additional evidence of early life on land around 3.5 billion years ago can be found in the Dresser formation of Australia in a hot spring facies, indicating that some of Earth's earliest life on land occurred in hot springs.[24] Microbially induced sedimentary structures (MISS) are found throughout the geologic record up to 3.2 billion years old. They are formed by the interaction of microbial mats and physical sediment dynamics, and record paleoenvironmental data as well as providing evidence of early life.[25] The different paleoenvironments of early life on Earth also serves as model when searching for potential fossil life on Mars.
Extremophiles
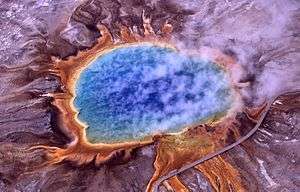
Another area of investigation in geomicrobiology is the study of extremophile organisms, the microorganisms that thrive in environments normally considered hostile to life. Such environments may include extremely hot (hot springs or mid-ocean ridge black smoker) environments, extremely saline environments, or even space environments such as Martian soil or comets.[4]
Observations and research in hyper-saline lagoon environments in Brazil and Australia as well as slightly saline, inland lake environments in NW China have shown that anaerobic sulfate-reducing bacteria may be directly involved in the formation of dolomite.[27] This suggests the alteration and replacement of limestone sediments by dolomitization in ancient rocks was possibly aided by ancestors to these anaerobic bacteria.[28]
In July 2019, a scientific study of Kidd Mine in Canada discovered sulfur-breathing organisms which live 7900 feet below the surface, and which breathe sulfur in order to survive. these organisms are also remarkable due to eating rocks such as pyrite as their regular food source. [29][30][31]
See also
- Bacterial oxidation
- Desulforudis audaxviator
- Deep biosphere
References
- Smith, H. E. K.; Tyrrell, T.; Charalampopoulou, A.; Dumousseaud, C.; Legge, O. J.; Birchenough, S.; Pettit, L. R.; Garley, R.; Hartman, S. E.; Hartman, M. C.; Sagoo, N.; Daniels, C. J.; Achterberg, E. P.; Hydes, D. J. (21 May 2012). "Predominance of heavily calcified coccolithophores at low CaCO3 saturation during winter in the Bay of Biscay". Proceedings of the National Academy of Sciences. 109 (23): 8845–8849. Bibcode:2012PNAS..109.8845S. doi:10.1073/pnas.1117508109. PMC 3384182. PMID 22615387.
- Gadd, GM (2010). "Metals, minerals and microbes: geomicrobiology and bioremediation". Microbiology. 156 (3): 609–43. doi:10.1099/mic.0.037143-0. PMID 20019082.
- U.S. Geological Survey (2007). "Facing tomorrow's challenges - U.S. Geological Survey science in the decade 2007-2017". U.S. Geological Survey Circular. 1309: 58.
- Konhauser, K. (2007). Introduction to geomicrobiology. Malden, MA: Blackwell Pub. ISBN 978-1444309027.
- Kaksonen, A.H.; Puhakka, J.A (2007). "Sulfate Reduction Based Bioprocesses for the Treatment of Acid Mine Drainage and the Recovery of Metals". Engineering in Life Sciences. 7 (6): 541–564. doi:10.1002/elsc.200720216.
- "Mitigation of Climate Change in Agriculture (MICCA) Programme | Food and Agriculture Organization of the United Nations". www.fao.org. Retrieved 2019-10-02.
- Canfield, D.E.; Kristensen, E.; Thamdrup, B. (2005). Aquatic geomicrobiology (Transferred to digital print ed.). London: Elsevier Acad. Press. ISBN 978-0121583408.
- Gray, C.J.; Engel, A.S. (2013). "Microbial diversity and impact on carbonate geochemistry across a changing geochemical gradient in a karst aquifer". The ISME Journal. 7 (2): 325–337. doi:10.1038/ismej.2012.105. PMC 3555096. PMID 23151637.
- Johnson, H.P.; Pruis, M.J. (2003). "Fluxes of Fluid and Heat from the Oceanic Crustal Reservoir". Earth and Planetary Science Letters. 216 (4): 565–574. Bibcode:2003E&PSL.216..565J. doi:10.1016/S0012-821X(03)00545-4.
- Smith, A.R.; Fisk, M.R.; Thurber, A.R; Flores, G.E.; Mason, O.U.; Popa, R.; Colwell, F.S. (2016). "Deep crustal communities of the Juan de Fuca ridge are governed by mineralogy". Geomicrobiology. 34 (2): 147–156. doi:10.1080/01490451.2016.1155001.
- Rawlings, D.E. (2005). "Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates". Microbial Cell Fact. 4 (13): 13. doi:10.1186/1475-2859-4-13. PMC 1142338. PMID 15877814.
- Colwell, F.S.; D'Hondt, S. (2013). "Nature and Extent of the Deep Biosphere". Reviews in Mineralogy and Geochemistry. 75 (1): 547–574. Bibcode:2013RvMG...75..547C. doi:10.2138/rmg.2013.75.17.
- Rajala, Pauliina; Bomberg, Malin; Vepsalainen, Mikko; Carpen, Leena (2017). "Microbial fouling and corrosion of carbon steel in deep anoxic alkaline groundwater". Biofouling. 33 (2): 195–209. doi:10.1080/08927014.2017.1285914. PMID 28198664.
- Cheung, K.H.; Gu, Ji-Dong (2007). "Mechanism of hexavalent chromium detoxification by microorganusms and bioremediation application potential: A review". International Biodeterioration & Biodegradation. 59: 8–15. doi:10.1016/j.ibiod.2006.05.002.
- Al-Battashi, H; Joshi, S.J.; Pracejus, B; Al-Ansari, A (2016). "The Geomicrobiology of Chromium (VI) Pollution: Microbial Diveristy and its Bioremediation Potential". The Open Biotechnology Journal. 10 (Suppl-2, M10): 379–389. doi:10.2174/1874070701610010379.
- Choppola, G; Bolan, N; Park, JH (2013). Chapter two: Chromium contamination and its risk assessment in complex environmental settings. Advances in Agronomy. 120. pp. 129–172. doi:10.1016/B978-0-12-407686-0.00002-6. ISBN 9780124076860.
- Luptakova, A; Kusnierova, M (2005). "Bioremediation of acid mine drainage contaminated by SRB". Hydrometallurgy. 77 (1–2): 97–102. doi:10.1016/j.hydromet.2004.10.019.
- Canfield, D.E (2001). "Biogeochemistry of Sulfur Isotopes". Reviews in Mineralogy and Geochemistry. 43 (1): 607–636. Bibcode:2001RvMG...43..607C. doi:10.2138/gsrmg.43.1.607.
- Leahy, J. G.; Colwell, R. R. (1990). "Microbial degradation of hydrocarbons in the environment". Microbiological Reviews. 54 (3): 305–315. PMC 372779. PMID 2215423.
- McCollom, Thomas M.; Christopher, Donaldson (2016). "Generation of hydrogen and methane during experimental low-temperature reaction of ultramafic rocks with water". Astrobiology. 16 (6): 389–406. Bibcode:2016AsBio..16..389M. doi:10.1089/ast.2015.1382. PMID 27267306.
- Onstott, T.C.; McGown, D.; Kessler, J.; Sherwood Lollar, B.; Lehmann, K.K.; Clifford, S.M. (2006). "Martian CH4: Sources, Flux, and Detection". Astrobiology. 6 (2): 377–395. Bibcode:2006AsBio...6..377O. doi:10.1089/ast.2006.6.377. PMID 16689653.
- Noffke, Nora (2007). "Microbially induced sedimentary structures in Archean sandstones: A new window into early life". Gondwana Research. 11 (3): 336–342. Bibcode:2007GondR..11..336N. doi:10.1016/j.gr.2006.10.004.
- Bontognali, T. R. R.; Sessions, A. L.; Allwood, A. C.; Fischer, W. W.; Grotzinger, J. P.; Summons, R. E.; Eiler, J. M. (2012). "Sulfur isotopes of organic matter preserved in 3.45-billion-year-old stromatolies reveal microbial metabolism". PNAS. 109 (38): 15146–15151. Bibcode:2012PNAS..10915146B. doi:10.1073/pnas.1207491109. PMC 3458326. PMID 22949693.
- Djokic, Tara; Van Kranendonk, Martin J.; Campbell, Kathleen A.; Walter, Malcolm R.; Ward, Colin R. (2017). "Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits". Nature Communications. 8: 15263. Bibcode:2017NatCo...815263D. doi:10.1038/ncomms15263. PMC 5436104. PMID 28486437.
- Noffke, Nora; Christian, Daniel; Wacey, David; Hazen, Robert M. (2013). "Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ca. 3.48 Billion-Year-Old Dresser Formation, Pilbara, Western Australia". Astrobiology. 13 (12): 1103–1124. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. PMC 3870916. PMID 24205812.
- Thomas D. Brock. "Colorful Yellowstone". Life at High Temperatures. Archived from the original on 2005-11-25.
- Deng, S; Dong, H; Hongchen, J; Bingsong, Y; Bishop, M (2010). "Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: results from Quighai Lake, Tibetan Plateau, NW China". Chemical Geology. 278 (3–4): 151–159. Bibcode:2010ChGeo.278..151D. doi:10.1016/j.chemgeo.2010.09.008.
- Dillon, Jesse (2011). The Role of Sulfate Reduction in Stromatolites and Microbial Mats: Ancient and Modern Perspectives. Stromatolites: Interaction of Microbes with Sediments. Cellular Origin, Life in Extreme Habitats and Astrobiology. 18. pp. 571–590. doi:10.1007/978-94-007-0397-1_25. ISBN 978-94-007-0396-4.
- Lollar, Garnet S.; Warr, Oliver; Telling, Jon; Osburn, Magdalena R.; Lollar, Barbara Sherwood (2019). "'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory". Geomicrobiology Journal. 36 (10): 859–872. doi:10.1080/01490451.2019.1641770.
- World’s Oldest Groundwater Supports Life Through Water-Rock Chemistry, July 29, 2019, deepcarbon.net.
- Strange life-forms found deep in a mine point to vast 'underground Galapagos', By Corey S. Powell, Sept. 7, 2019, nbcnews.com.
Further reading
- Ehrlich, Henry Lutz; Newman, Dianne K., eds. (2008). Geomicrobiology (5th ed.). Hoboken: Taylor & Francis Ltd. ISBN 978-0849379079.
- Jain, Sudhir K.; Khan, Abdul Arif; Rai, Mahendra K. (2010). Geomicrobiology. Enfield, NH: Science Publishers. ISBN 978-1439845103.
- Kirchman, David L. (2012). Processes in microbial ecology. Oxford: Oxford University Press. ISBN 978-0199586936.
- Loy, Alexander; Mandl, Martin; Barton, Larry L., eds. (2010). Geomicrobiology molecular and environmental perspective. Dordrecht: Springer. ISBN 978-9048192045.
- Nagina, Parmar; Ajay, Singh, eds. (2014). Geomicrobiology and Biogeochemistry. Berlin, Heidelberg: Springer Berlin Heidelberg. ISBN 978-3642418372.
