Euryoryzomys russatus
Euryoryzomys russatus, also known as the russet oryzomys,[3] russet rice rat,[1] or big-headed rice rat,[4] is a species of rodent in the family Cricetidae. It is a member of the genus Euryoryzomys, which was split off from Oryzomys in 2006. It was first described by Johann Andreas Wagner in 1848.[1] It is found in southern Brazil,[5] eastern Paraguay[5] and northeastern Argentina.[5] It is considered a large species in its genus, with a reddish-brown coat, long tail length, and large skull.[5] It is a terrestrial rodent, spending its time foraging for seeds, fruits, and insects.[6][7] It is listed by the IUCN as least concern, although studies have shown it to be influenced by anthropogenic disturbances.[8] Predators consist of small members of the order Carnivora.[9][10]
| Euryoryzomys russatus | |
|---|---|
 | |
| Holotype partial cranium of Calomys coronatus Winge, 1887, a junior synonym | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Rodentia |
| Family: | Cricetidae |
| Subfamily: | Sigmodontinae |
| Genus: | Euryoryzomys |
| Species: | E. russatus |
| Binomial name | |
| Euryoryzomys russatus | |
| Synonyms | |
|
Calomys coronatus Winge, 1887 | |
Etymology
The prefix eury- comes from the Greek word 'eurys' meaning "wide" or "broad".[11] The specific epithet russatus comes from the Latin word 'russatus', meaning "clothed in red".[12]
Taxonomy
Euryoryzomys russatus[13] (Wagner, 1848) is the currently accepted name for the russet rice rat. It is a member of the order Rodentia and family Cricetidae[14] with the genus Euryoryzomys comprising six valid species.[15]
Distribution and habitat
Euryoryzomys russatus is found in continental Brazil and outlying islands.[5] This species is also found in Bolivia, Argentina, and Paraguay.[5] It is found along altitudinal gradients consisting of lowland and mountainous (montane) areas.[5][16] It is found in the Atlantic Forest as well as some areas of the Amazon rainforest.[5][16][17]
Specific locations where E. russatus has been collected include: Desterro Environmental Conservation Unit central Santa Catarina Island, Brazil (live specimens);[18] Alem Paraiba, Minas Gerais, Brazil (museum specimens);[16] Guaricana National Park, Paraná, Brazil (museum specimens);[16] Ilha do Cardoso, São Paulo, Brazil (museum specimens);[16] Parana, Southern Brazil (genetic identification from scat of predatory felines);[9] Picinguaba, São Paulo, Brazil (live specimens);[19] Rio de Janeiro, Brazil (live specimens);[20] Morro Grande Forest Reserve, São Paulo, Brazil (live specimens).[21]
This species is considered a forest specialist, dwelling only in habitats that have extensive forest canopy cover.[22] Abundance and immigration rates have been shown to increase as forest coverage increases.[22]
Life history
.png)
Morphology
The genus Euryoryzomys is described as having pelage that varies from yellow in color to a red-brown dorsally, while having a lighter ventral color.[23] The ears are typically medium to large. Vibrissae (whiskers) do not extend past the ears. Most species possess a jugal (with the exception of E. lamia).[23]
Euryoryzomys russatus is described as having a large skull and relatively large body length; with a body length range of 112–185 mm (4.4–7.3 in).[5] Weights have been recorded in some studies, and averaged 59 g (2.1 oz).[21] The tail averages between 105–196 mm (4.1–7.7 in) in length.[5] Pelage of the rice rat is a reddish-brown on the dorsal portion of the body and white on the ventral portion. The pinnae and tail are both grey in color. The fore and hindlimbs are a pale pink, along with the nose. The facial vibrissae are black in color. Parietals in E. russatus possess no lateral expansions.[23]
Ecology
Euryoryzomys russatus is a nocturnal, terrestrial rodent that moves primarily over leaf litter found on the forest floor.[21][24] A seasonal microhabitat selection study found variation in microhabitat choice in warm-wet and cool-dry seasons.[21] Euryoryzomys russatus was shown to have greater abundance in areas with woody debris, low leaf litter height, and high arthropod biomass during the warm-wet season and during the cool-dry season the greatest abundance was seen in areas with high leaf litter humidity.[21] It is an opportunistic eater, consuming seeds, fruit, and insects when possible.[6][7] A study of seed predation in the Brazilian Atlantic forest found E. russatus to be an efficient seed predator, eating a majority of seeds offered (with an exception to those with a mass greater than that of the observed individuals).[25]
A study of the population dynamics of a population of E. russatus on Santa Catarina Island in southern Brazil showed them to have nearly equal sex ratios.[18] Populations of E. russatus have been shown to have a monogamous mating system.[7][18] Females show reproductive activity throughout the year, and correlate with availability of food resources.[7] The nests of E. russatus are cup-shaped, and built with fibers from bamboo and other grasses from the family Poaceae.[24] Offspring are born altricial, lacking hair, and with eyes and ears closed.[24] A study tracking individuals of E. russatus calculated the average number of offspring per pregnancy (from survey of pregnant females and nestlings) to be 3.6, with three to six nestlings being typical.[24]
Genetics
Genetic analyses[17] have grouped different populations of E. russatus into three clades using mitochondrial and nuclear gene regions, however no subspecies have been identified.[17] A study focused on the genetic structuring of populations in the Atlantic Rain Forest of southern Brazil found no genetic structuring throughout the species distribution.[26] Through karyotyping of E. russatus individuals from Parque Estadual da Serra do Mar (Santa Virginia, Brazil) it was found that they possess a chromosome number of 2n=80.[14] This number is shared with E. emmonsae and E. nitidus.[14]
Conservation
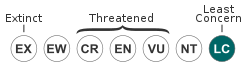
Euryoryzomys russatus is listed as a species of least concern by the IUCN as of September 2016.[8] However, studies have shown this species to be susceptible to anthropogenic disturbances such as habitat degradation or destruction.[17][22][27][28]
Predators
Studies have found the E. russatus to be a prey item of several neotropical feline species including jaguarundi (Puma yagouaroundi), oncilla (Leopardus tigrinus) and ocelot (Leopardus pardalis).[9] Domestic cats (Felis silvestris catus) have also been shown to prey on E. russatus.[10]
Parasites
Research into the gastrointestinal parasites of E. russatus found eight different endoparasites in island and continental populations.[29] A new species of nematode, Hassalstrongylus luquei, was found in the small intestine of E. russatus.[20] A single individual was found to possess antibodies from a systemic fungal infection with Paracoccidioides brasiliensis.[30] A study on Rickettsia rickettsii, Brazilian Spotted-fever has shown E. russatus to be a host for the tick species Amblyomma ovale, which is a known vector for the zoonotic disease.[31]
References
- Percequillo, A.; Weksler, M.; Langguth, A.; Patterson, B.; D'Elia, G.; Teta, P. (2016). "Euryoryzomys russatus (errata version published in 2017)". The IUCN Red List of Threatened Species. 2016: e.T29405A115168400. doi:10.2305/IUCN.UK.2016-3.RLTS.T29405A22328896.en.
- Wagner, Andr (1848). "Beiträge zur Kenntniss der Säugthiere Amerika's". Abhandlungen der Mathematisch-Physikalischen Klasse der Königlich Bayerischen Akademie der Wissenschaften. München. 5 (2): 312.
- Musser, G.G.; Carleton, M.D. (2005). "Superfamily Muroidea". In Wilson, D.E.; Reeder, D.M (eds.). Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. p. 1154. ISBN 978-0-8018-8221-0. OCLC 62265494.
- Duff, A. and Lawson, A. 2004. Mammals of the World: A checklist. Yale University Press, 312 pp. ISBN 978-0-300-10398-4
- Patton, James; Pardinas, Ulyses; D'Elia, Guillermo (2015). Mammals of South America: Rodents. 2. Chicago: University of Chicago Press. p. 319.
- Powers AM, Mercer DR, Watts DM, Guzman H, Fulhorst CF, Popov VL and Tesh RB (1999) Isolation and genetic characterization of a hantavirus (Bunyaviridae: Hantavirus) from a rodent, Oligoryzomys microtis (Muridae), collected in northeastern Peru Am J Trop Med Hyg 61:92–98
- Bergallo, H. & Magnusson, W. (2004). Factors affecting the use of space by two rodent species in Brazilian Atlantic forest. Mammalia. 68. 121-132. 10.1515/mamm.2004.013.
- Percequillo, A.; Weksler M.; Langguth, A.; Patterson, B.; D'Elia, G.; Teta, P. (2016). "Euryoryzomys russatus (errata version published in 2017)". IUCN Red List of Threatened Species. 2016: e.T29405A115168400. doi:10.2305/IUCN.UK.2016-3.RLTS.T29405A22328896.en. Retrieved 12 January 2020.{{cite iucn}}: error: |doi= / |page= mismatch (help)
- Silva-Pereiraa, J. E.; Moro-Rios, R. F.; Bilski, D. R.; Passos, F. C. (2010). "Diets of three sympatric Neotropical small cats: Food niche overlap and interspecies differences in prey consumption". Mammalian Biology. 76 (3): 308–312. doi:10.1016/j.mambio.2010.09.001.
- Ferreira, Giovanne Ambrosio; Nakano-Oliveira, Eduardo; Genaro, Gelson (2014). "Domestic cat predation on Neotropical species in an insular Atlantic Forest remnant in southeastern Brazil". Wildlife Biology. 20 (3): 167–175. doi:10.2981/wlb.13131.
- "eury- | Origin and meaning of prefix eury- by Online Etymology Dictionary". www.etymonline.com. Retrieved 2019-09-28.
- "Definition of russatus". Latin Lexicon. Retrieved 2019-09-28.
- Wagner, A. J. (1848). Beiträge zur Kenntniss der Säugthiere Amerika's / vom A. Wagner. Munich, Germany: Königliche Akademie der Wissenschaften. pp. 271–332. doi:10.5962/bhl.title.15738.
- Di-Nizo, C. B.; Neves, C. L.; Vilela, J. F.; Silva, M. J. (2014-01-24). "New karyologycal data and cytotaxonomic considerations on small mammals from Santa Virgínia (Parque Estadual da Serra do Mar, Atlantic Forest, Brazil)". Comparative Cytogenetics. 8 (1): 11–30. doi:10.3897/compcytogen.v8i1.6430. ISSN 1993-078X. PMC 3978240. PMID 24744831.
- Patton, J. L.; Pardiñas, U. F. J.; D’Elía, G. (2015). Mammals of South America, Volume 2. University of Chicago Press. pp. 314–321. doi:10.7208/chicago/9780226169606.001.0001. ISBN 9780226169576.
- Libardi, G. S.; Percequillo, A. R. (2016). "Variation of craniodental traits in russet rats Euryoryzomys russatus (Wagner, 1848) (Rodentia: Cricetidae: Sigmodontinae) from Eastern Atlantic Forest". Zoologischer Anzeiger: 57–74.
- Miranda, Gustavo B.; Andrades-Miranda, Jaqueline; Oliveira, Luiz F. B.; Langguth, Alfredo; Mattevi, Margarete S. (2007). "Geographic Patterns of Genetic Variation and Conservation Consequences in Three South American Rodents". Biochemical Genetics. 45 (11–12): 839–856. doi:10.1007/s10528-007-9122-x. PMID 17939030.
- Antunes, P., Campos, M., Oliveira-Santos, L., & Graipel, M. (2009). Population dynamics of Euryoryzomys russatus and Oligoryzomys nigripes (Rodentia, Cricetidae) in an Atlantic forest area, Santa Catarina Island, Southern Brazil. Biothemes, 22 (2), 143-151. doi: https://doi.org/10.5007/2175-7925.2009v22n2p143
- Pinheiro, P.S.; Geise, L. (2008). "Non-volant mammals of Picinguaba, Ubatuba, state of São Paulo, southeastern Brazil". Biol. Mus. Biol. Mello Leitao (N. Ser). 23: 51–59.
- Costa, M.A.R., Maldonado Jr, A., Bóia, M.N., Lucio, C.S. & Simões, R.O. 2014. a new species of Hassalstrongylus (Nematoda: Heligmonelidae) from Euryoryzomys russatus (Rodentia: Sigmodontinae) in the Atlantic forest, Brazil. Neotropical Helminthology, vol. 8, n°2, jul-dec, pp. 235-242.
- Naxara, L., Pinotti, B., & Pardini, R. (2009). Seasonal Microhabitat Selection by Terrestrial Rodents in an Old-Growth Atlantic Forest. Journal of Mammalogy, 90(2), 404–415. Retrieved from http://www.jstor.org/stable/30224486
- Püttker, T. A. A. Bueno, C. Santos de Barros, S. Sommer, R. Pardini. 2013. Habitat specialization interacts with habitat amount to determine dispersal success of rodents in fragmented landscapes, Journal of Mammalogy, Volume 94, Issue 3, 11:714–726, https://doi.org/10.1644/12-MAMM-A-119.1
- Weksler, M.; Percequillo, A. R.; Voss, R. S. (2006-10-19). "Ten new genera of oryzomyine rodents (Cricetidae: Sigmodontinae)" (PDF). American Museum Novitates. American Museum of Natural History. 3537: 1–29.
- Ricardo S. Bovendorp, Jessica A. Laskowski and Alexandre R. Percequillo 2016. A first view of the unseen: nests of an endangered Atlantic Forest rat species. Mammalia (Short Note): 1-4. DOI 10.1515/mammalia-2015-0178
- Galetti, M.; Guevara, R.; Galbiati, L. A.; Neves, C. L.; Rodarte, R. R.; Mendes, C. P. (2015). "Seed Predation by Rodent and Implications for Plant Recruitment in Defaunated Atlantic Forests". Biotropica. 47 (5): 521–525. doi:10.1111/btp.12246.
- Gonçalves, Gislene L.; Marinho, Jorge R.; Freitas, Thales R.O. (2009). "Genetic structure of sigmodontine rodents (Cricetidae) along an altitudinal gradient of the Atlantic Rain Forest in southern Brazil". Genetics and Molecular Biology. 32 (4): 882–885. doi:10.1590/S1415-47572009005000081. PMC 3036879. PMID 21637469.
- Pardini R, Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv 124:253–266
- Umetsu F, Pardini R (2007) Small mammals in a mosaic of forest remnants and anthropogenic habitats evaluating matrix quality in an Atlantic forest landscape. Landscape Ecol 22:517–530
- Kuhnen, VV.; Graipel, ME.; Pinto, CJC. (2012). "Differences in richness and composition of gastrointestinal parasites of small rodents (Cricetidae, Rodentia) in a continental and insular area of the Atlantic Forest in Santa Catarina state, Brazil". Brazilian Journal of Biology. 72 (3): 563–567. doi:10.1590/S1519-69842012000300019. PMID 22990827.
- Sbeghen, Mônica Raquel; Zanata, Thais Bastos; MacAgnan, Rafaela; De Abreu, Kaue Cachuba; Da Cunha, Willian Luiz; Watanabe, Maria Angelica Ehara; De Camargo, Zoilo Pires; Ono, Mario Augusto (2015). "Paracoccidioides brasiliensis Infection in Small Wild Mammals". Mycopathologia. 180 (5–6): 435–440. doi:10.1007/s11046-015-9928-8. PMID 26232125.
- Szabó, Matias P. J.; Pinter, Adriano; Labruna, Marcelo B. (2013). "Ecology, biology and distribution of spotted-fever tick vectors in Brazil". Frontiers in Cellular and Infection Microbiology. 3: 27. doi:10.3389/fcimb.2013.00027. PMC 3709097. PMID 23875178.