Erlenmeyer–Plöchl azlactone and amino-acid synthesis
The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone (also known as an azlactone).[1][2]
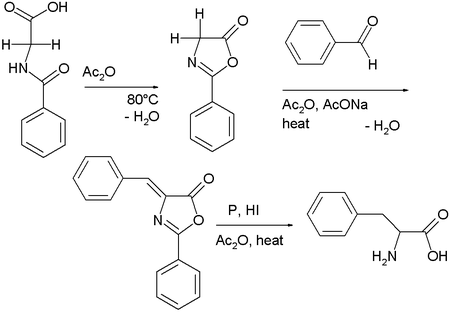
Hippuric acid, the benzamide derivative of glycine, cyclizes in the presence of acetic anhydride, condensing to give 2-phenyl-oxazolone.[3] This intermediate also has two acidic protons and reacts with benzaldehyde, acetic anhydride and sodium acetate to a so-called azlactone. This compound on reduction gives access to phenylalanine.[4]
Variations
Variants of the azlactone synthesis in which analogues of azlactones are used are sometimes advantageous. Hydantoin (in Bergmann modification), thiohydantoin and rhodanine have each been employed as the enolate-forming component of the condensation. [5][6] 2,5-Diketopiperazine can be used as a methylene component as well; its condensation products with aromatic aldehydes, on reduction and hydrolysis give the corresponding amino acids. [7][8][9]
Scope
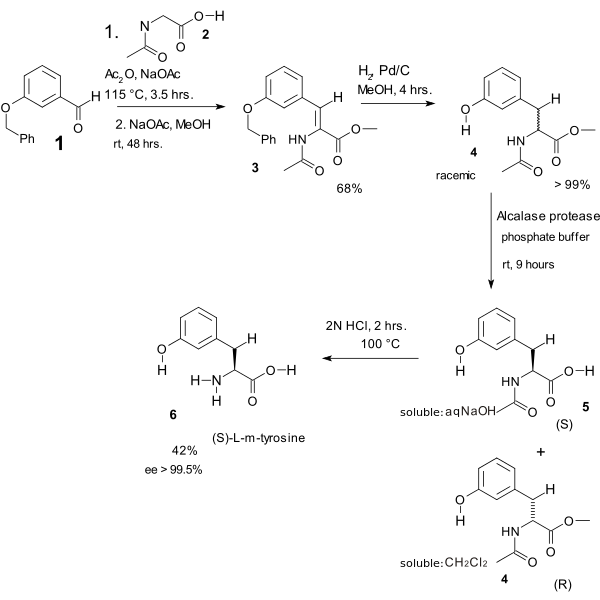
In one study the Erlenmeyer amino acid synthesis was used in the heart of an L-m-tyrosine synthesis [10][11]
See also
- Dakin-West reaction
- Perkin reaction
References
- Plöchl, J. (1884). "Über einige Derivate der Benzoylimidozimtsäure" [On some derivatives of benzoyl-imido-cinnaminic acid]. Berichte der deutschen chemischen Gesellschaft. 17: 1616–1624. ; see especially pp. 1623-1624.
- Erlenmeyer, F. (1893). "Ueber die Condensation der Hippursäure mit Phtalsäureanhydrid und mit Benzaldehyd" [On the condensation of hippuric acid with phthalic acid anhydride and with benzaldehyde]. Annalen der Chemie. 275: 1–8. doi:10.1002/jlac.18932750102. ; see especially pp. 3-8.
- G. E. VandenBerg, J. B. Harrison, H. E. Carter, B. J. Magerlein (1973). "2-Phenyl-2-oxazolone". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 5, p. 946
- H. B. Gillespie, H. R. Snyder (1934). "dl-β-Phenylalanine". Organic Syntheses.; Collective Volume, 2, p. 489
- Alfred Hassner, Irishi Namboothiri (2011). Organic Syntheses Based on Name Reactions: A Practical Guide to 750 Transformations. Elsevier. p. 139.
- Richard O.C. Norman, James M. Coxon (1993). Principles of Organic Synthesis (3rd ed.). CRC Press. p. 219–220.
- H.D. Dakin. Aromatic aldehyde derivatives of proteins, peptides and amino acids. J. Biol. Chem. 1929, 84:675-682
- Alan D. Borthwick. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. DrugMolDesign, 15 Temple Grove, London NW11 7UA, U.K. Chem. Rev., 2012, 112 (7), pp 3641–3716. DOI: 10.1021/cr200398y
- A. M. Asiri. New Conjugated Systems Derived from Piperazine-2,5-dione. Molecules 2000, 5, 629-636
- Cara E. Humphrey, Markus Furegati, Kurt Laumen, Luigi La Vecchia, Thomas Leutert, J. Constanze D. Müller-Hartwieg, and Markus Vögtle (2007). "Optimized Synthesis of L-m-Tyrosine Suitable for Chemical Scale-Up". Organic Process Research & Development. 11 (6): 1069–1075. doi:10.1021/op700093y.CS1 maint: multiple names: authors list (link)
- The benzyl ether of 3-hydroxybenzaldehyde 1 reacts with the N-acetyl amide of glycine 2, acetic anhydride and sodium acetate to the azlactone (not displayed) which is ring-opened with sodium acetate in methanol to dehydroamino acid 3. Hydrogenation gives the N-acyl-m-tyrosine methyl ester 4 (the benzyl ether group is also cleaved). This compound is racemic and kinetic resolution is brought about by an enzyme which is able to only cleave the methyl ester of the S-enantiomer (forming (S)-5 soluble in dichloromethane) leaving water soluble (R)-4 untouched. The final step is amide cleavage to (S)-L-m-tyrosine 6