Perkin reaction
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid.[1][2] The alkali salt acts as a base catalyst, and other bases can be used instead.[3]

The Perkin reaction
| Perkin reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | William Henry Perkin | ||||||||||
| Reaction type | Condensation reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
| Identifiers | |||||||||||
| RSC ontology ID | RXNO:0000003 | ||||||||||
| | |||||||||||
Reaction mechanism
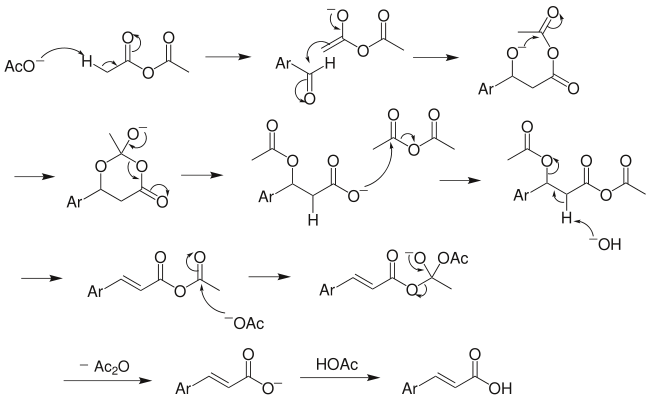
The Perkin reaction
The above mechanism is not universally accepted, as several other versions exist, including decarboxylation without acetic group transfer.[7]
Applications
- One notable application for the Perkin reaction is in the laboratory synthesis of the phytoestrogenic stilbene resveratrol (c.f. fo-ti).[8]
gollark: GOOØØØØØØØØØØØØØØÐÐС¡¡¡
gollark: <@!711651678260166678>
gollark: <@711651678260166678>
gollark: 19:00 BST next year.
gollark: ++remind 10y Specifically the "mind shaft" one of 2020.
See also
- Erlenmeyer–Plöchl azlactone and amino-acid synthesis
- Stobbe condensation
- Pechmann condensation
References
- Perkin, W. H. (1868). "On the artificial production of coumarin and formation of its homologues". Journal of the Chemical Society. 21: 53–61. doi:10.1039/js8682100053.
- Perkin, W. H. (1877). "On some hydrocarbons obtained from the homologues of cinnamic acid; and on anethol and its homologues". Journal of the Chemical Society. 32: 660–674. doi:10.1039/js8773200660.
- Dippy, J. F. J.; Evans, R. M. (1950). "The nature of the catalyst in the Perkin condensation". J. Org. Chem. 15 (3): 451–456. doi:10.1021/jo01149a001.
- Johnson, J. R. (1942). "The Perkin Reaction and Related Reactions". Org. React. 1: 210. doi:10.1002/0471264180.or001.08. ISBN 0471264180.
- House, H. O. (1972) Modern Synthetic Reactions, W. A. Benjamin, Menlo Park, California, 2nd ed, pp. 660–663
- Rosen, T. (1991). "The Perkin Reaction". Compr. Org. Synth. 2: 395–408. doi:10.1016/B978-0-08-052349-1.00034-2. ISBN 978-0-08-052349-1.
- Bansal, Raj K. (1998) Organic Reaction Mechanisms, Tata McGraw Hill, 3rd Edition , pp. 199–201, ISBN 9780470858585 doi:10.1002/0470858583.
- Solladié, Guy; Pasturel-Jacopé, Yacine; Maignan, Jean (2003). "A re-investigation of resveratrol synthesis by Perkins reaction. Application to the synthesis of aryl cinnamic acids". Tetrahedron. 59 (18): 3315–3321. doi:10.1016/S0040-4020(03)00405-8. ISSN 0040-4020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.