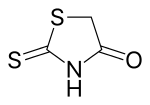
Rhodanine
Rhodanine is a 5-membered heterocyclic organic compound possessing a thiazolidine core. It was discovered in 1877 by Marceli Nencki who named it "Rhodaninsaure" in reference to its synthesis from ammonium rhodanide (known as ammonium thiocyanate to modern chemists) and chloroacetic acid in water.[3]
 | |
| Names | |
|---|---|
| IUPAC name
2-Sulfanylidene-1,3-thiazolidin-4-one | |
| Other names
2-Thioxo-4-thiazolidinone; 4-Oxo-2-thioxothiazoline | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.005 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H3NOS2 | |
| Molar mass | 133.18 g·mol−1 |
| Density | 0.868 g/cm−3[2] |
| Melting point | 170 °C (338 °F; 443 K)[2] |
| Soluble[2] | |
| Solubility | Ethanol, dimethyl sulfoxide[2] |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R22 R41 |
| S-phrases (outdated) | S22 S26 S39 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
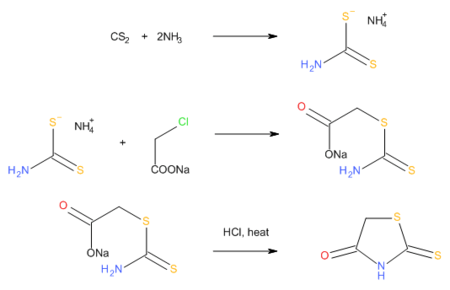
Rhodanines can also be prepared by the reaction of carbon disulfide, ammonia, and chloroacetic acid, which proceeds via an intermediate dithiocarbamate.[4]
Derivatives
Some rhodanine derivatives have pharmacological properties; for instance, epalrestat is used to treat diabetic neuropathy. However, most are promiscuous binders with poor selectivity; as a result, this class of compounds is viewed with suspicion by medicinal chemists.[5][6][7] However, this view is discussed (both correct use of PAINS filters and necessity of the experimental confirmations of such properties) as well as many useful features of rhodanine derivatives.[8][9]
References
- Rhodanine at Sigma-Aldrich
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 3.512. ISBN 978-1439855119.
- Nencki, M. (10 July 1877). "Ueber die Einwirkung der Monochloressigsäure auf Sulfocyansäure und ihre Salze". Journal für Praktische Chemie. 16 (1): 1–17. doi:10.1002/prac.18770160101.
- Redemann, C. Ernst; Icke, Roland N.; Alles, Gordon A. (1955). "Rhodanine". Organic Syntheses.; Collective Volume, 3, p. 763
- Baell, J. B; Holloway, G. A (2010). "New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays". J. Med. Chem. 53 (7): 2719–2740. CiteSeerX 10.1.1.394.9155. doi:10.1021/jm901137j. PMID 20131845.
- Tomašić, Tihomir; Peterlin Mašič, Lucija (2012). "Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation". Expert Opinion on Drug Discovery. 7 (7): 549–60. doi:10.1517/17460441.2012.688743. PMID 22607309.
- Pouliot, Martin; Jeanmart, Stephane (8 September 2015). "Pan Assay Interference Compounds (PAINS) and Other Promiscuous Compounds in Antifungal Research". Journal of Medicinal Chemistry. 59 (2): 497–503. doi:10.1021/acs.jmedchem.5b00361. PMID 26313340.
- Kaminskyy, D; Kryshchyshyn, A; Lesyk, R (2017). "Recent developments with rhodanine as a scaffold for drug discovery". Expert Opinion on Drug Discovery. 12 (12): 1233–1252. doi:10.1080/17460441.2017.1388370. PMID 29019278.
- Kaminskyy, D; Kryshchyshyn, A; Lesyk, R (2017). "5-Ene-4-thiazolidinoneseAn efficient tool in medicinal chemistry". Eur. J. Med. Chem. 140 (10): 542–594. doi:10.1016/j.ejmech.2017.09.031. PMC 7111298. PMID 28987611.