Cardiovirus
Cardiovirus are a group of viruses within order Picornavirales, family Picornaviridae. Vertebrates serve as natural hosts for these viruses.[1]
| Cardiovirus | |
|---|---|
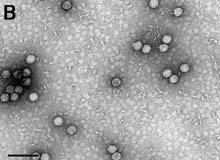 | |
| TEM micrograph of Cardiovirus A virions bar scale equals 100 nm | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Picornavirales |
| Family: | Picornaviridae |
| Genus: | Cardiovirus |
| Type species | |
| Cardiovirus A | |
Taxonomy
There are currently six species in the genus. In addition to the type species, Cardiovirus A, there is Cardiovirus B, Cardiovirus C, Cardiovirus D, Cardiovirus E, and Cardiovirus F. Diseases associated with cardioviruses include: myocarditis, encephalitis, multiple sclerosis, and type 1 diabetes.[1][2][3]
Cardiovirus A is composed of only one serotype, encephalomycarditis virus (EMCV). Cardiovirus B consists of four viruses that are most probably serologically distinct. These are Theiler's Murine encephalomyelitis virus (TMEV), Vilyuisk human encephalomyelitis virus (VHEV), a Theiler-like rat virus (TRV) (which has yet to be named) and Saffold virus (SAF-V). Of these 4, only VHEV and SAF-V are thought to cause infection in humans.[4] Thus far, cardiovirus C has only been observed in the brown rat.[4][5]
Structure
Cardioviruses are single-stranded RNA, non-enveloped viruses with icosahedral or spherical geometries, and a T=pseudo3 icosahedral capsid protein geometry. The diameter is around 30 nm. Genomes are linear and non-segmented, around 7.8 kb in length.[2][6] The T=pseudo3 icosahedral capsid of Cadiovirus is made of 60 protomers, each of which contains 4 polypeptides: VP1, VP2, VP3, and VP4.[7][8] Inside the capsid of the virus there is a single linear, positive single-stranded RNA. On both the 3' and 5' ends of the genome there are untranslated regions .[8] These untranslated regions of genome are important for the DNA replication, with the untranslated region on the 5' side being the location of internal ribosomal entry site (IRES).[8]
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Cardiovirus | Icosahedral | Pseudo T=3 | Non-enveloped | Linear | Monopartite |
Life cycle
Viral replication is cytoplasmic. Entry into the host cell is achieved by attachment of the virus to host receptors, which mediates endocytosis. Replication follows the positive stranded RNA virus replication model. Positive stranded rna virus transcription is the method of transcription. Translation takes place by -1 ribosomal frameshifting, viral initiation, and ribosomal skipping. The virus exits the host cell by lysis, and viroporins. Human and vertebrates serve as the natural host. Transmission routes are zoonosis and fomite.[2]
The 3’ end of the genome encodes a polyA tail while the 5’ end encodes a genome-linked protein. A unique feature of this genus is the presence of the L* protein, 18kDa, that is made out of frame from the polyprotein and is present in the DA subgroup of TMEV.[9] It has been found to be important for the virus pathogenesis.
In the case of Cardiovirus A, the virus can cause encephalitis and myocarditis, mostly in rodents, which are natural hosts. The virus is transmitted from rodents to other animals. Severe epidemics have been seen in swine and elephants.[10]
Replication of cardioviruses is dependent on a structured RNA element called the Cardiovirus cis-acting replication element (CRE).
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Cardiovirus | Humans; vertebrates | Gastrointestinal tract; CNS; heart | Cell receptor endocytosis | Lysis | Cytoplasm | Cytoplasm | Zoonosis; fomite |
Clinical
Human cardioviruses were first isolated in 1981. Seven additional isolates have since been described in North America, Europe and South Asia. They have been associated with gastroenteritis, influenza-like symptoms and non-polio-associated acute flaccid paralysis. The first infection of cardiovirus in humans was identified in 2007 in a stool sample of an infant that was experiencing fever of unknown origin.[6] It was subsequently named the Saffold virus after the lead researcher, Morris Saffold Jones.[6]
Other pathogenic cardioviruses isolated from humans include the Syr-Darya valley fever virus and Vilyuisk human encephalomyelitis virus.[11]
See also
- Saffold virus (Cardiovirus B)
- Theiler's encephalomyelitis virus
References
- Blinkova, Olga; Kapoor, Amit; Victoria, Joseph; Jones, Morris; Wolfe, Nathan; Naeem, Asif; Shaukat, Shahzad; Sharif, Salmaan; Alam, Muhammad Masroor; Angez, Mehar; Zaidi, Sohail (1 May 2009). "Cardioviruses Are Genetically Diverse and Cause Common Enteric Infections in South Asian Children". Journal of Virology. 83 (9): 4631–4641. doi:10.1128/JVI.02085-08. ISSN 0022-538X. PMC 2668475. PMID 19193786.
- "Viral Zone". ExPASy. Retrieved 15 June 2015.
- "Virus Taxonomy: 2019 Release". talk.ictvonline.org. International Committee on Taxonomy of Viruses. Retrieved 7 May 2020.
- Wang, Yan; Zhao, Jing; Zheng, Min; Liu, Zhijian; Li, Wang; Fu, Xingli; Lin, Yuan; Yuan, Jiaqi; Zhao, Jieji; Shen, Quan; Wang, Xiaochun (27 March 2018). "A novel cardiovirus in wild rats". Virology Journal. 15 (1): 58. doi:10.1186/s12985-018-0968-9. ISSN 1743-422X. PMC 5872539. PMID 29587779.
- Jones, MS.; Lukashov, VV; Ganac, RD; Schnurr, DP. (July 2007). "Discovery of a novel Human Picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin". Journal of Clinical Microbiology. 45 (7): 2144–2150. doi:10.1128/JCM.00174-07. PMC 1933019. PMID 17460053.
- Himeda, Toshiki; Ohara, Yoshiro (February 2012). "Saffold Virus, a Novel Human Cardiovirus with Unknown Pathogenicity". Journal of Virology. 86 (3): 1292–1296. doi:10.1128/JVI.06087-11. ISSN 0022-538X. PMC 3264344. PMID 22114344.
- "Cardiovirus ~ ViralZone page". viralzone.expasy.org. Retrieved 1 May 2020.
- Mullapudi, Edukondalu; Nováček, Jiří; Pálková, Lenka; Kulich, Pavel; Lindberg, A. Michael; van Kuppeveld, Frank J. M.; Plevka, Pavel (12 August 2016). "Structure and Genome Release Mechanism of the Human Cardiovirus Saffold Virus 3". Journal of Virology. 90 (17): 7628–7639. doi:10.1128/JVI.00746-16. ISSN 0022-538X. PMC 4988150. PMID 27279624.
- Tan, Shawn Zheng Kai; Tan, Mark Zheng Yi; Prabakaran, Mookkan (January 2017). "Saffold virus, an emerging human cardiovirus". Reviews in Medical Virology. 27 (1): e1908. doi:10.1002/rmv.1908. ISSN 1052-9276. PMC 7169152. PMID 27723176.
- Fenner FJ.; Gibbs EPJ; Murphy FA; Rott R; Studdert MJ; White, DO (1993). Veterinary Virology (2nd ed.). Academic Press, Inc. ISBN 0-12-253056-X.
- Anonymous. (2014) Genetic characterization of the Syr-Darya valley fever virus (SDVFV) (Picornaviridae, Cardiovirus) isolated from the blood of the patients and ticks Hyalomma as. asiaticum (Hyalomminae), Dermacentor daghestanicus (Rhipicephalinae) (Ixodidae) and Ornithodoros coniceps (Argasidae) in Kazakhstan and Turkmenistan. Vopr Virusol 59(4):15-19